All News
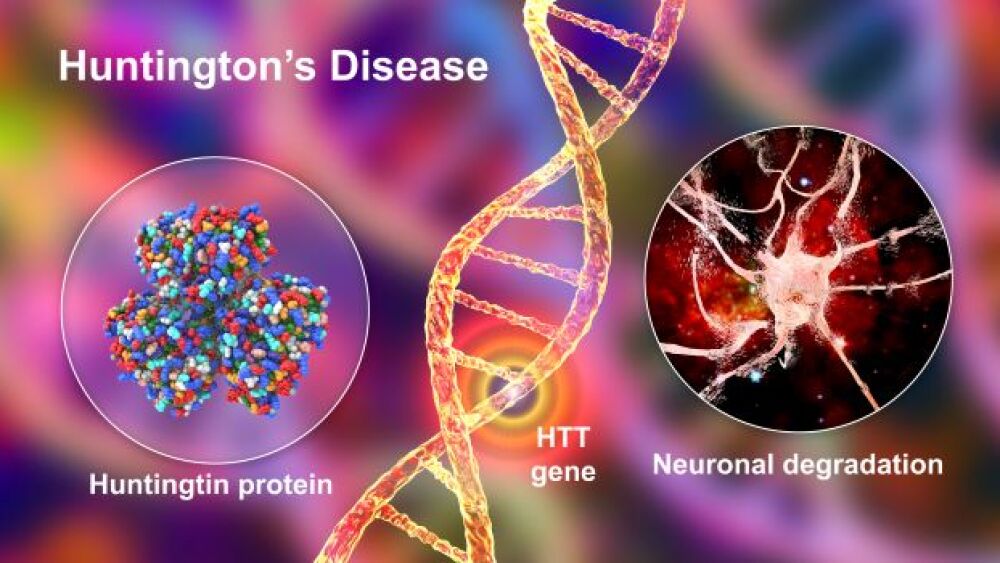
Annexon released final data from an open-label phase II clinical trial, showing that its drug candidate ANX005 safely stabilized disease progression in patients with Huntington’s disease.
The Tisch MS Research Center of New York has been researching the concept of using cell therapy as a therapeutic strategy to promote repair and regeneration in certain patients with MS.
On Tuesday, the FDA’s Vaccines and Related Biological Products Advisory Committee voted 21-0 with one abstention to recommend authorization of Novavax’s COVID-19 vaccine.
Code Bio, a biotechnology company leading the way in targeted non-viral delivery of generic medicines, closed a $75 million Series A financing round on Tuesday.
For the first time in half a decade, the FDA will convene to address two therapies developed by bluebird bio that could have ripple effects across the industry regarding lentiviral vectors.
Aslan partners with Johns Hopkins and Duke, Editas joins forces with Immatics, Yumanity (soon to be Kineta, Inc.) teams with Janssen, Serotiny & Janssen Biotech and Ginkgo and Novo Nordisk.
Amarin and Vincerx Pharma intend to restructure their financial strategies and lessen operational costs by trimming their employee rosters.
Seres Therapeutics, Inc. announced that the company’s Phase III Ecospor IV study demonstrated a strong safety profile in addition to statistically significant positive results against C. difficile infection.
The Phase I mechanism of action study, showing that Lilly’s injectable Mounjaro (tirzepatide) induces greater weight loss than placebo and Novo Nordisk’s semaglutide in adults with type 2 diabetes.
Powered by its blockbuster checkpoint inhibitor Keytruda, Merck is forecasting a potential of more than 80 new regulatory approvals in oncology through 2028.
The Phase III ADvocate 1 and 2 studies on lebrikizumab showed that eight out of 10 patients with atopic dermatitis maintained skin clearance in the 12 months they had been under treatment.
Novartis has fallen victim to a cyberattack. The Swiss pharma giant’s data was hijacked by a well-known online extortion ring dubbed Industrial Spy.
BioSpace caught up with Tara Frenkl and Nelson Ambrogio ahead of ASCO to discuss Bayer’s goal of becoming a top 10 oncology company by 2030.
CEL-SCI Corporation, Jazz Pharmaceuticals, CARsgen and Ascentage Pharma presented clinical trial data at the American Society of Clinical Oncology 2022 Annual Meeting.
Sernova shared positive Phase I/II data from an ongoing clinical study of its implantable Cell Pouch device that showed evidence of in vivo active insulin production.
Results of a Phase II/III study showed that patients who were given Tafinlar plus Mekinist saw an overall response rate of 47% vs. 11% for those who received standard chemotherapy only.
Johnson & Johnson and Emergent BioSolutions are accusing each other of breaching their COVID-19 vaccine supply agreement.
The Phase II/III Aria Study was unsuccessful in achieving statistical significance on the primary endpoint or any secondary objectives, according to Praxis.
In a small study of 14 rectal cancer patients, researchers at Memorial Sloan Kettering Cancer Center published results where 100% of the 12 patients who completed treatment went into remission.
The combination of Opdivo plus Yervoy in addition to chemotherapy demonstrated additional benefits in patient populations who usually have poor prognoses.