All News
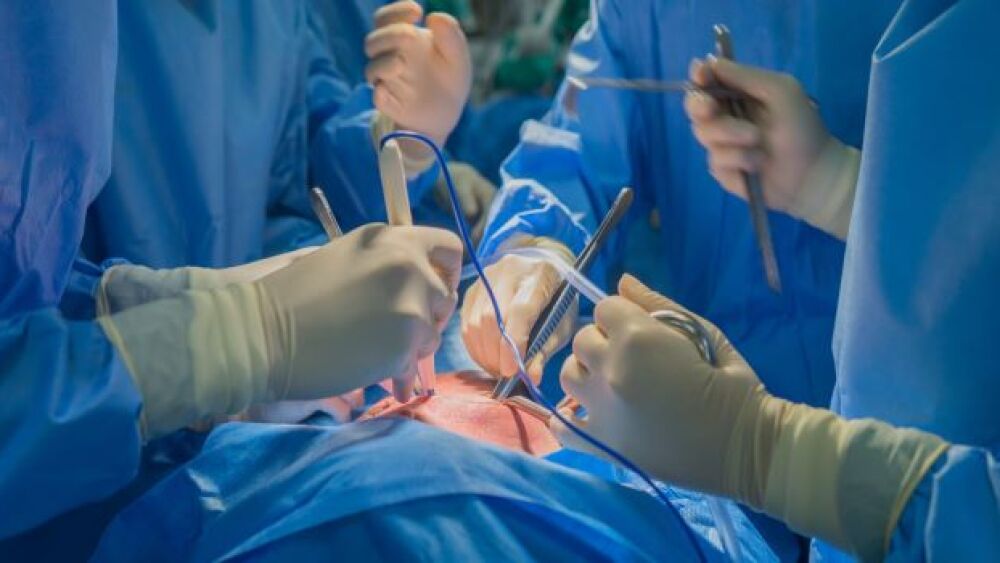
Physicians at New York University have successfully implanted pig hearts into the bodies of patients that were living but no longer showed neural function, a process called xenotransplantation.
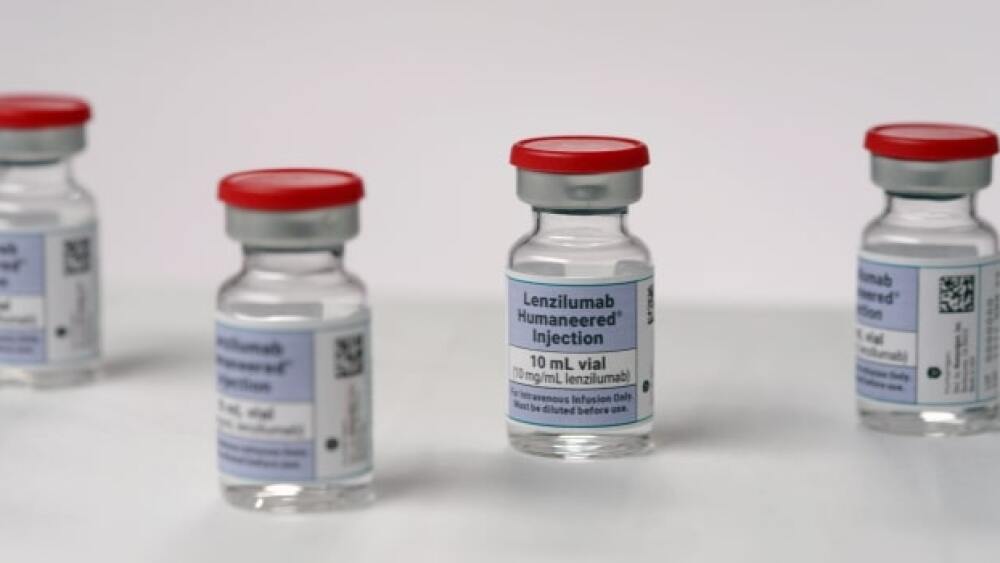
An NIH-sponsored trial assessing a combination of Humanigen’s lenzilumab and Gilead Sciences’ remdesivir in hospitalized COVID-19 patients failed to achieve the primary endpoint.
Sanofi, Certara, Pfizer and Thermo Fisher are facing hiring challenges head-on with a common strategy: upskilling programs. They use these programs to fill open roles while training and retaining existing talent.
In what seems like an unlikely collaboration, Seattle-based Fred Hutchinson Cancer Center (Fred Hutch) is partnering with Amazon to develop cancer vaccines.
Atara announced that it has completed an interim analysis of its Phase II EMBOLD trial for the use of its candidate ATA188 in patients with progressive multiple sclerosis.
The pace of unicorn creation – which is still quite fast – is slowing as economists predict a coming recession. BioSpace sought out the perspective of four experts.
Aldeyra Therapeutics announced results from a crossover clinical trial, showing that its drug candidate reproxalap was effective in improving ocular redness and tear production in patients with dry eye disease.
Moderna has dosed its first participant in a Phase I clinical trial of mRNA-1215, a vaccine designed to fight the Nipah virus (NiV), a virus contracted in humans through animals.
Annexon and Pliant Therapeutics both made efforts to secure additional financing to support their R&D endeavors.
Backed by biopharma royalty, Areteia Therapeutics will focus on developing an old molecule for a very prevalent disease - asthma.
Genentech has exercised the second option in its potentially $1.7 billion agreement with Bicycle Therapeutics, triggering a $10 million payment.
Nanoscope Therapeutics will make two presentations at the 2022 ASRS meeting on their gene therapy programs to treat degenerative retinal diseases, retinitis pigmentosa and Stargardt disease.
Verve Therapeutics dosed its first patient with VERVE-101, a one-time treatment that aims to permanently lower low-density lipoprotein (LDL) cholesterol in patients and prevent heart disease.
Epic Bio aims to transform genetic medicine by developing a new class of drugs that target the epigenome to alter gene expression.
Flagship Pioneering has launched Apriori Bio, which utilizes artificial intelligence to predict virus activity and variant formation, including COVID-19.
uniQure gave a 12-month update on AMT-130, the first-ever AAV gene therapy for HD to enter clinical trials. BioSpace spoke with the company’s president of R&D, Dr. Ricardo Dolmetsch.
Exelixis’ Cabometyx, in combination with two BMS-owned drugs, hit the primary endpoint of progression-free survival but missed the secondary endpoint of overall survival in renal cell carcinoma.
Roundworms are the cornerstone of scientific discoveries that have helped shape how we understand human disease and treatment. BioSpace takes a look at advances in C. elegans research.
Vertex Pharmaceuticals plans to acquire ViaCyte for $320 million in cash. ViaCyte focuses on stem cell-derived cell replacement therapies that they hope will cure type 1 diabetes (T1D).
Tonix Pharmaceuticals announced a mid-stage study for major depressive disorder, which followed the June disclosure of a Phase II study for a cocaine intoxication therapy.