Regulatory
The FDA has placed a clinical hold on Beam Therapeutics’ leukemia/lymphoma therapy and has lifted the hold on Celyad’s CAR-T candidate for colorectal cancer.
The FDA granted Priority Review for omidubicel with a target action date of January 30, 2023. Omidubicel is a first-in-class, advanced NAM (nicotinamide)-enabled stem cell therapy.
The end of July is busy for the FDA, with Coherus, Sanofi, Acadia, Myovant and Pfizer having PDUFA dates filling the calendar. Here’s a look.
InMed updated its INM-755 for patients with epidermolysis bullosa, Seagen and Astellas announced positive topline results for Padcev with Merck’s Keytruda, and more.
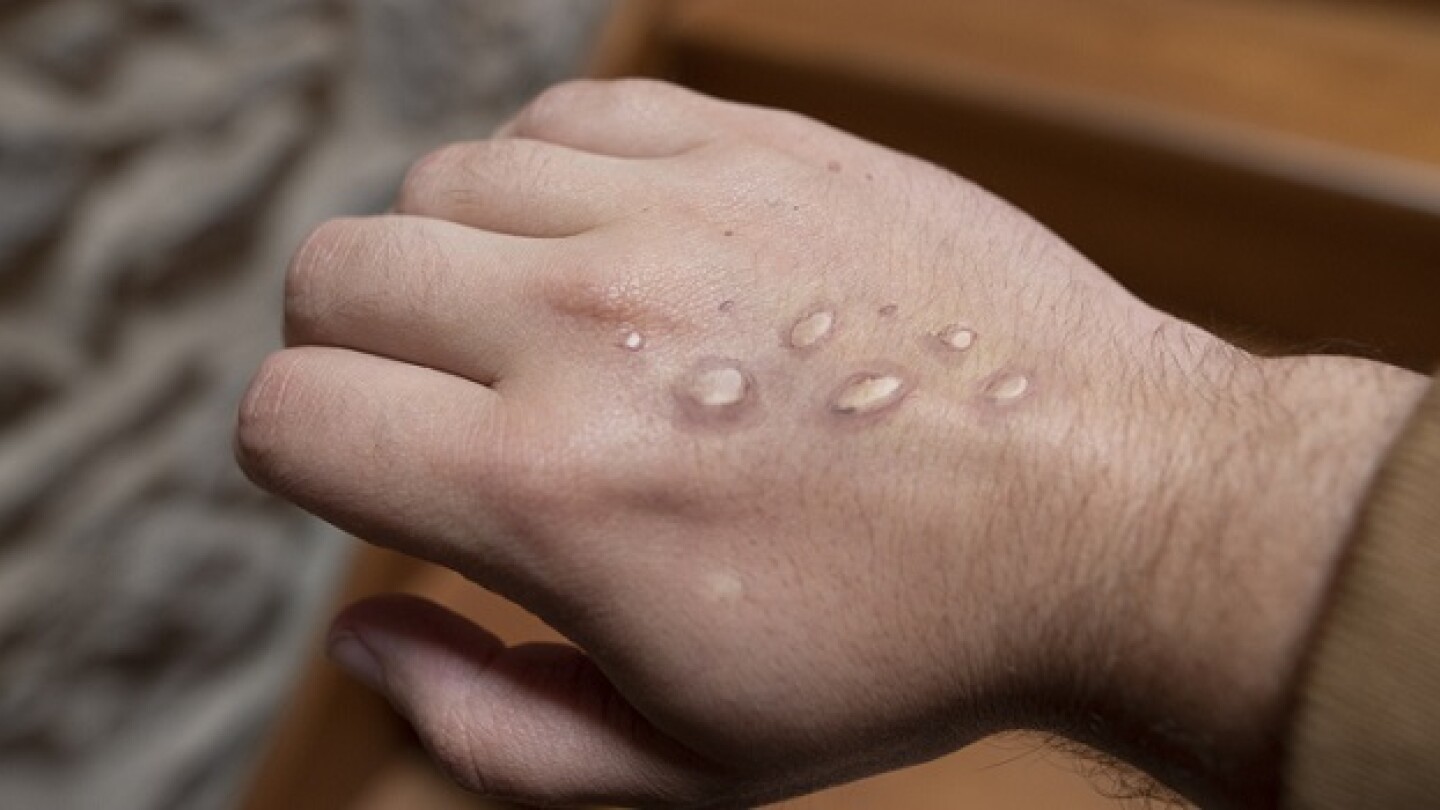
WHO declared the monkeypox outbreak a “public health emergency of international concern [PHEIC].” As COVID-19 wanes, Tonix, SIGA, Emergent Bio and others are now targeting monkeypox.
The complaint alleges the agencies made an error under the Administrative Procedure Act, violating the Food Drug and Cosmetics Act by failing to approve Lumryz.
FDA Weekly Review looks at the FDA’s actions related to drug approvals, IND approvals, designations and more. Here’s a look at what happened this week.
Sandoz hopes its high-concentration formulation of an already greenlit biosimilar will be approved by the time AbbVie’s blockbuster drug Humira loses patent protection in the United States.
In what it is calling a strategic decision, Sesen Bio announced Monday that it has paused development activities of Vicineum, its lead asset, in the United States.
Mid-July is a relatively quiet time for the FDA in terms of drug approvals, but Incyte and Arcutis will have all eyes on the regulator as they await decisions in vitiligo and psoriasis.
PRESS RELEASES