Phase II
Moderna is poised to commercialize its COVID-19 and influenza combination vaccine by the fall of 2023, with some potential for the same drug to also be viable against the RSV.
The agency recommended another clinical trial be conducted to confirm the data from the study on the 3.2-mg dose of the intranasal carbetocin for Prader-Willi syndrome.
Amagma Therapeutics announced a licensing agreement with Innovent Biologics for up to three enzyme specific inhibitors derived from Amagma’s proprietary SEIZMIC Platform.
The company will continue to analyze data regarding the secondary endpoints.
The decision to carry on came after dosing for the Phase III GENERATION HD1 trial was halted following an overall risk/benefit assessment.
The problem Kinaset is trying to solve is that over time, patients receiving asthma therapies – typically inhaled corticosteroids plus bronchodilators – become steroid-refractory.
Unlike antibody-drug conjugates (ADCs) that focus on antigens, PDCs are able to target the tumor microenvironment that has universal features across solid tumors.
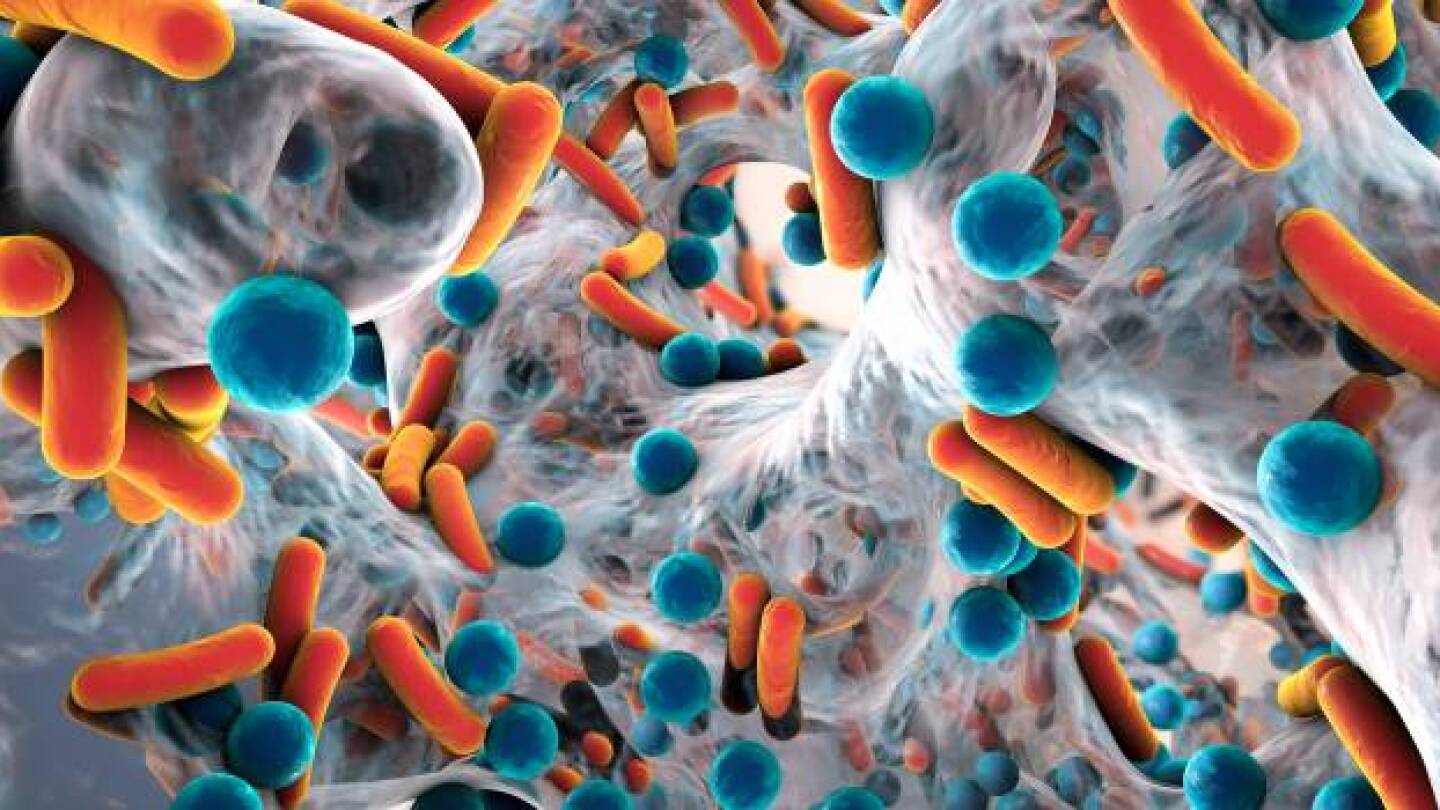
To alleviate the problem of antibiotic-resistant bacteria, or superbugs, several biotechnology companies are developing different strategies to target and kill these microorganisms.
Affini-T Therapeutics unveiled its mission to target oncogenic driver mutations to deliver transformative therapies intended to cure patients.
Heading into the middle of January, companies announced plenty of new clinical trial news. Here’s a look.
PRESS RELEASES