Food and Drug Administration (FDA)
NEWS
The planned testing for DNL919 was held back pending additional documentation required to initiate the studies and information on the preclinical toxicology assessment.
Eli Lilly’s proposed antibody drug bebtelovimab has received EUA from the FDA after demonstrating its potency against SARS-CoV-2’s Omicron variant.
Pfizer indicated it will wait for the full data on a three-dose series of the vaccine for that population, believing it “may provide a higher level of protection in this age group.
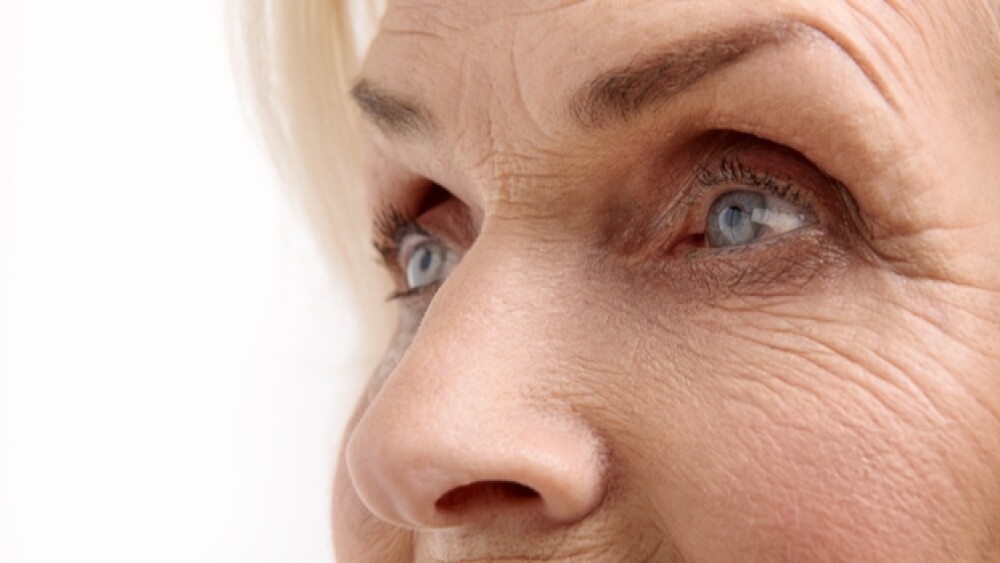
New two-year data from Phase III studies of Susvimo and Vabysmo reinforce the two drugs’ potential to improve the quality of life of patients diagnosed with DME and nAMD.
CMS issued a draft decision for Biogen’s Alzheimer’s drug and said it would only cover the cost of Aduhelm and any required scans “if they are enrolled in qualifying clinical trials.”
The promising results from the pediatric expansion are what fuels Novavax’s next move to apply for regulatory approval for the 12-to-17-year age group by the first quarter of 2022.
The EUA request is based on the Phase II study that showed treatment with ensovibep reduced the viral load from the SARS-CoV-2 virus within eight days of use compared to placebo.
Merck has announced positive results from its Phase III KEYNOTE-522 study on the use of KEYTRUDA alongside chemotherapy in high-risk early-stage triple-negative breast cancer.
The FDA is slowing down the process of potentially approving dozens of new medications initially developed for the Chinese market.
JOBS
IN THE PRESS