Food and Drug Administration (FDA)
NEWS
Innovation Pharmaceuticals, a clinical stage pharmaceutical company, is pleased to inform shareholders the Company received notification from the Food and Drug Administration that a waiver has been granted eliminating the need to study Brilacidin, for the prevention of Severe Oral Mucositis in Head and Neck Cancer patients receiving chemoradiation, in pediatric populations.
Shares of specialty pharmacy company Aquestive Therapeutics are up more than 18% in premarket trading following a late Friday afternoon approval from the U.S. FDA for Exservan, a treatment for amyotrophic lateral sclerosis.
The latest approval for Calquence, a Bruton tyrosine kinase inhibitor, was granted under the FDA’s Real-Time Oncology Review and newly established Project Orbis programs.
The approval marks the first time a Korean company independently brought a drug from discovery to FDA approval.
MC2 Therapeutics announced that the U.S. Food and Drug Administration has accepted for review the New Drug Application for Wynzora™ Cream.
The U.S. Food and Drug Administration approved Alnylam Pharmaceuticals’ Givlaari (givosiran) for acute hepatic porphyria.
Levo Therapeutics, Inc. announced that the U.S. Food and Drug Administration has granted Fast Track designation for LV-101 for the treatment of Prader-Willi syndrome Levo is currently enrolling participants in its Phase 3 clinical study of intranasal carbetocin for the treatment of PWS.
AbbVie’s blockbuster drug Humira just got another challenger to its share of the market in the United States when its patent expires in 2023.
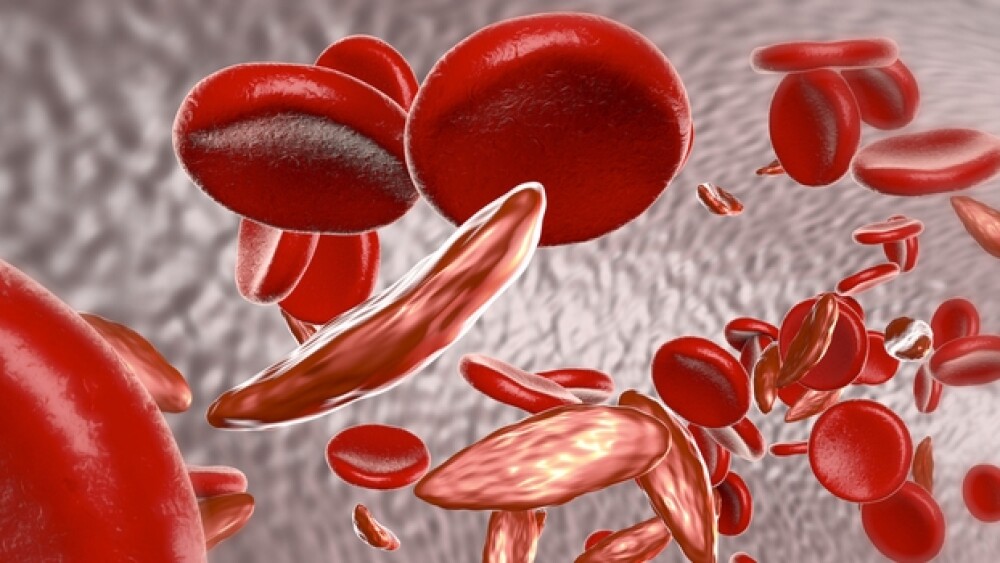
“The approval of Adakveo marks a new era in the treatment of sickle cell disease, a genetic condition that places an extraordinary burden of unpredictable pain crises on patients and their families,” said Susanne Schaffert, president of Novartis Oncology.
JOBS
IN THE PRESS