Pfizer
NEWS
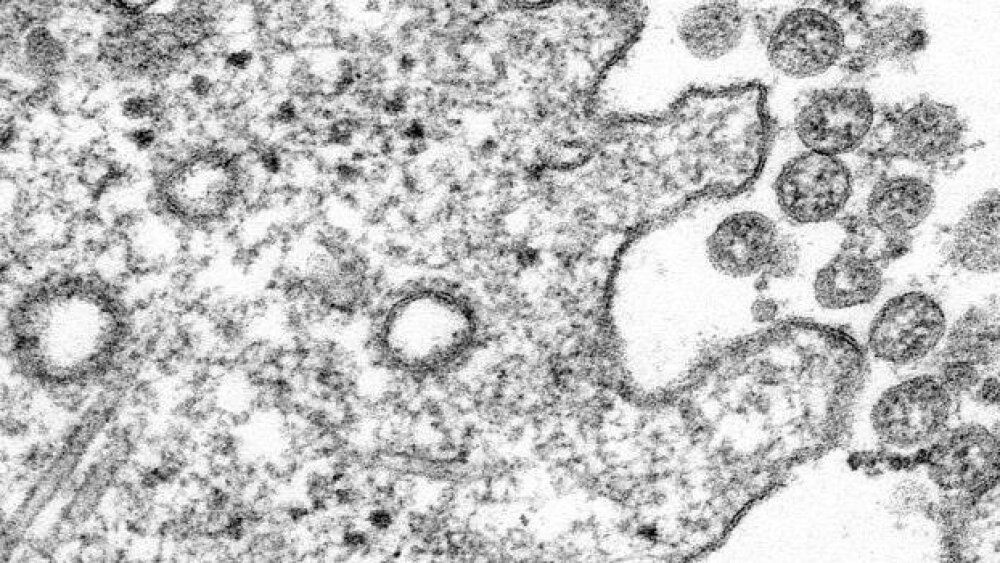
Please check out the biopharma industry coronavirus (COVID-19) stories that are trending for October 20, 2020.
Pfizer is continuing to invest in gene therapy research and development and other areas going on throughout the company, specifically in its North Carolina sites in Chapel Hill and Kit Creek.
It was another busy week for both COVID-19-related clinical trial news as well as trial updates for other indications. Here’s a look.
Pfizer will not seek Emergency Use Authorization for its COVID-19 vaccine until the end of November even if the readout from a Phase III study expected later this month is positive.
Several biopharmaceutical companies have made headway as of late in the race to develop a vaccine for COVID-19, which is responsible for the current global pandemic.
To date, all the major clinical trials of vaccines against COVID-19 have been in adults.
It was a moderately busy week for clinical trial news. Here’s a look.
This morning, the pharma giant announced the PENELOPE-B study failed to hit the mark of improved invasive disease-free survival (iDFS).
Results from multiple Phase III trials show consistent improvements in pain relief and physical function.
JOBS
IN THE PRESS