Johnson & Johnson Family of Companies
NEWS
So far during the first six months of the year, more than $100 billion has been spent on a myriad of acquisitions across the pharma and biotech industries.
Cambridge, Massachusetts-based Biogen exercised its option to acquire additional shares of Samsung Bioepis, a joint venture with Samsung BioLogics that was founded in 2012.
The Dementia Discovery Fund (DDF), a venture capital fund focused solely on dementia-related investments, completed $350 million of fundraising. The initial target was $200 million.
The week of June 18 to June 22 was bookended by a big hiring announcement in the pharma industry. It began with a former Cowen analyst being tapped as chief financial officer of startup Allogene and ended with the retirement announcement of the group worldwide chairman at Johnson & Johnson.
After waiting with bated breath, Johnson & Johnson Innovation finally opened its New York City JLABS facility with 26 resident companies, including the four winners of the JLABS @ NYC QuickFire Challenge.
The Biotechnology Institute gave its newly launched 2018 Woman of the Year Award to Seema Kumar.
Boston-based Emulate closed on a Series C round worth $36 million.
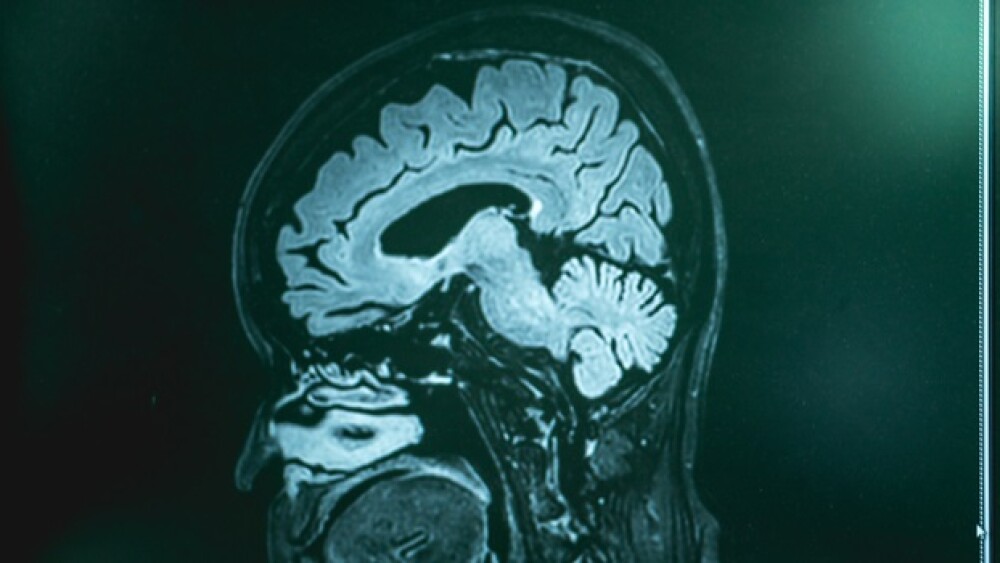
June is Alzheimer’s & Brain Awareness Month and June 21 is dubbed “The Longest Day,” which focuses on raising awareness of Alzheimer’s disease.
Not only are women generally underrepresented in the biopharma industry, but compared to many of the top male executives, underpaid. Here’s a closer look at the top five female executives by pay in the biopharma industry.
JOBS
IN THE PRESS