Clinical research
Despite not hitting endpoints, Cytokinetics noted that the FORTITUDE-ALS trial demonstrated consistency of effect for doses, endpoints and timepoints.
SMA is a severe neuromuscular disease caused by a mutation in the SMN1 gene, which codes for SMN, a protein necessary for motor neuron function.
Less than one year after securing a toehold in the United States, Korea-based Enzychem Lifesciences hit a major milestone with its pipeline as it eyes completion of its Phase II CRIOM (Chemoradiation Induced Oral Mucositis) study, which could lead to eventual commercialization of a treatment for the serious cancer treatment by-product.
The annual meeting of the American Society of Gene & Cell Therapy (ASGCT) was held in Washington, DC this week, with literally hundreds of abstracts and presentations. Here’s a look at some of the highlights.
When major depressive disorder (MDD) does not respond to at least two different types of antidepressant treatments in a moderate-to-severe depressive episode, it is reclassified as treatment-resistant depression or TRD. South San Francisco-based VistaGen Therapeutics’ AV-101 failed to meet its primary efficacy endpoints for TRD in a Phase II clinical trial.
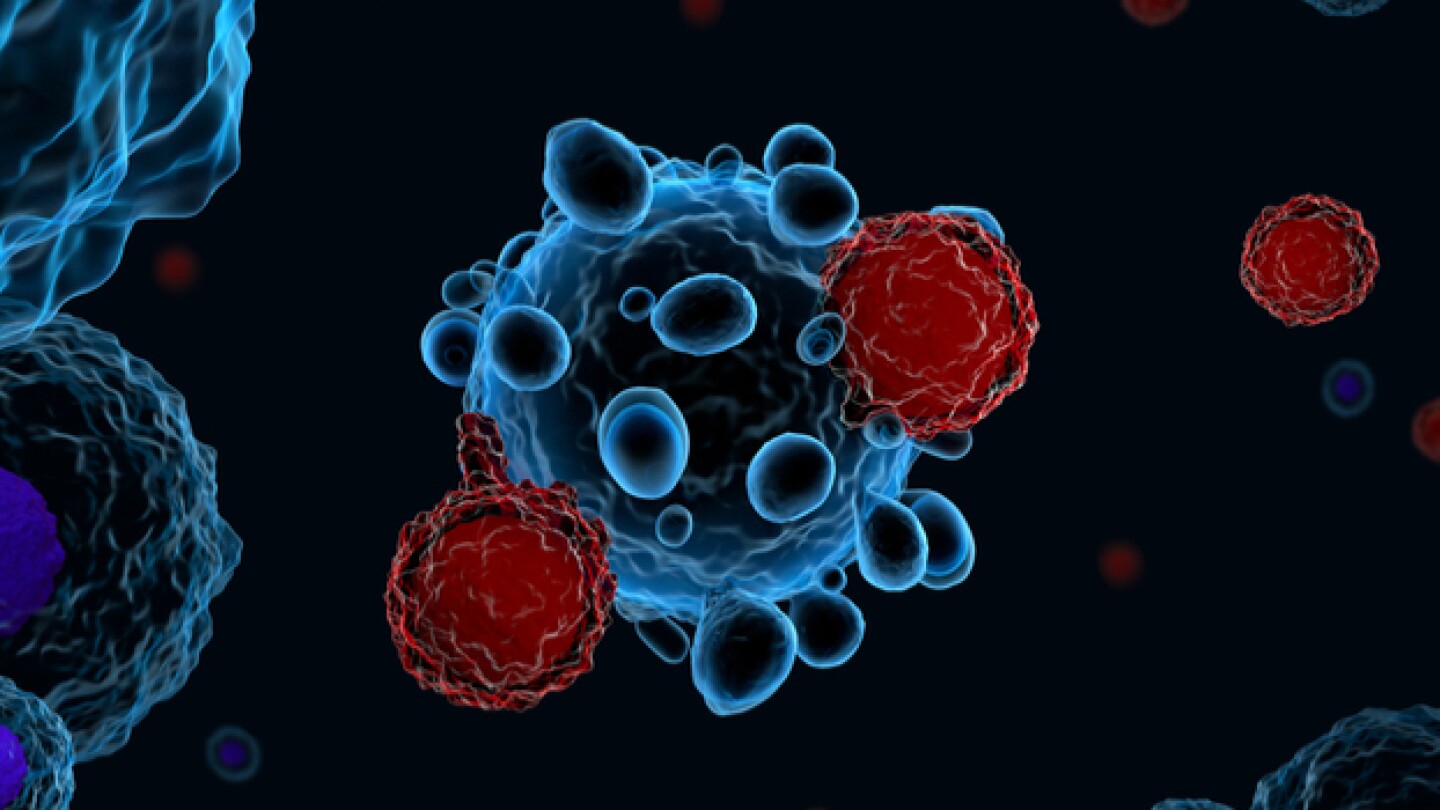
Celgene Corporation and bluebird bio published interim data from CRB-401, their Phase I trial of bb2121, their CAR-T therapy in patients with relapsed and refractory multiple myeloma.
In its announcement, GSK said the decision to cut the two programs were made following an analysis of available research results, including interim data from an ongoing Phase I study of the flu vaccine.
Collaboration covers proposed trastuzumab biosimilar in Phase III development for human epidermal growth factor receptor 2 positive (HER2+) breast and gastric tumors
Pharma, biotech and life science companies from across the globe share data and progress on pipelines and other company events.
Up to 6 patients to be enrolled in the second cohort, initial data expected in fourth quarter 2019
PRESS RELEASES