All News
Robert Califf’s nomination to resume his role as FDA Commissioner continues to advance in the Senate, despite some opposition from a key Democrat, Sen. Joe Manchin.
Bristol Myers Squibb and Exelixis reported two-year follow-up data from the Phase III trial of BMS’s checkpoint inhibitor Opdivo (nivolumab) and Exelixis’ Cabometyx (cabozantinib).
The unspoken challenge is: how diverse can a trial run in the United States really be right now?
The three-year agreement leverages AvantGen’s yeast display technology for antibody discovery optimization to engineering antibodies into Antibody-Drug Conjugate treatments.
The grant is the latest in a steady stream of company advances highlighted by the addition of a chief financial officer in January and several collaborative agreements in the past year.
Roivant Sciences launches new subsidiary Hemavant that will develop Eisai-licensed asset for transfusion-dependent anemia in patients with low-risk MDS.
With EVADER, the Chameleon team hopes to make one-and-done treatments a relic of the past.
Six months after the one-time treatment, 47% of patients in Cohort 1 demonstrated a promising two-step or greater improvement in their diabetic retinopathy.
Since 2016 the state has offered more than $70 million in tax credits to 114 different entities and has generated about $140 million in investments into the state’s life sciences ecosystem.
CMS says it would only cover the cost of Biogen’s Aduhelm and any required scans “if they are enrolled in qualifying clinical trials.”
ProQR Therapeutics has reported that their drug sepofarsen failed to meet its primary and secondary endpoints in recent Phase II/III clinical trials.
Stoughton, Mass.-based Collegium Pharmaceutical is expanding its pain-treating portfolio with a $604 million buyout of Raleigh’s BioDelivery Sciences International (BDSI).
The planned testing for DNL919 was held back pending additional documentation required to initiate the studies and information on the preclinical toxicology assessment.
Discover the most in-demand pharma qualifications and how to use them to score your dream job in our comprehensive guide.
Moderna is reportedly in talks with the UK government to establish mRNA manufacturing facilities in the country, as well as collaborating with health authorities on clinical trials.
Although the Omicron surge appears to be waning in the U.S., the World Health Organization keeps an eye on four Omicron subvariants.
Eli Lilly’s proposed antibody drug bebtelovimab has received EUA from the FDA after demonstrating its potency against SARS-CoV-2’s Omicron variant.
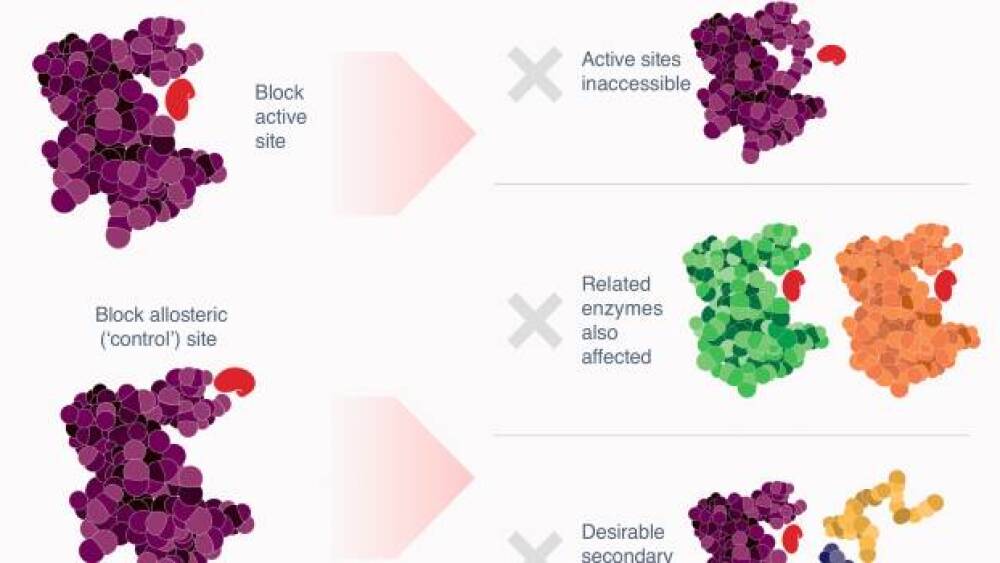
Exo Therapeutics’ approach is not only different, it has revolutionary potential.
Pfizer indicated it will wait for the full data on a three-dose series of the vaccine for that population, believing it “may provide a higher level of protection in this age group.
The fund will be used to target partnerships with promising early-stage biotechnology to enhance Fujifilm’s life sciences portfolio.