All News
Virtual Science AI announces the launch of Virtual Advisory AI, a virtual engagement platform designed to deliver the most effortless advisory board engagement experiences and the industry’s first AI-driven analytics following life science advisory board activities
The charges come from several in-process research and development projects acquired outside of a business combination.
In honor of World Hemophilia Day, BioSpace takes a look at some of the facts relating to the disease, and some of the highlights in recent hemophilia research and development.
The Chinese government has halted the recommendation of Lianhua Qingwen used as a traditional Chinese treatment for mild cases of COVID-19.
Thinking about starting a career in biotech? We’ve put together a guide to help you decide where to go within the biotech field and what steps you should take to get there.
There are very few ongoing clinical trials on treatments for Long COVID and there has been what appears to be a successful treatment using Pfizer’s Paxlovid. Read on for more details.
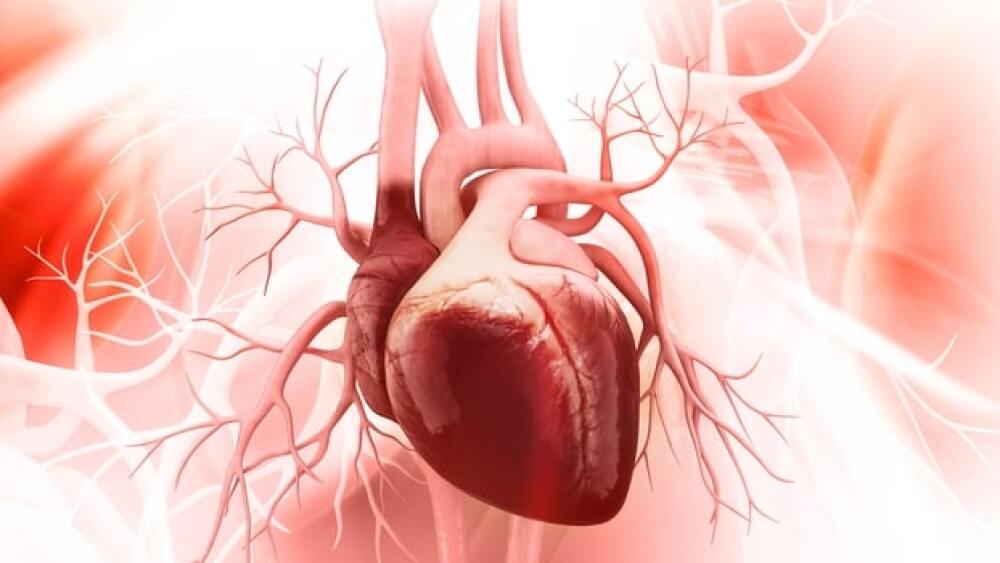
The university’s most recent discovery utilizes messenger RNA (mRNA) technology to combat the physiological effects of heart attacks – with potential for a near reversal of heart muscle damage.
Scientists recently announced the complete sequencing of the entire human genome. Biotech company PacBio - and HiFi sequencing - played a big role in this milestone.
TG Therapeutics voluntarily withdrew its pending BLA and supplemental NDA for its treatment dubbed U2 for adults with chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL).
It was a very busy week for clinical trial news, with much of it coming out of the American Association of Cancer Research Annual Meeting. Read on for details.
McKinsey & Company is under fire for allegedly allowing its employees to work simultaneously for big pharma companies and serve as consultants for the U.S. Food and Drug Administration.
There are an estimated 2.3 million people living with Multiple Sclerosis and hundreds of thousands more undiagnosed, adding up to an annual cost of nearly $85 billion for care in the United States.
The U.S.Food and Drug Administration has granted Emergency Use Authorization to a breathalyzer that can detect COVID-19 within three minutes.
The SEC added 12 China companies to their delisting watchlist this week. The companies will need to release evidence by May 3 to remain listed.
Regeneron announced that the U.S. FDA has extended its review of the BLA for monoclonal antibody REGEN-COV, which treats COVID-19.
The search for a biopharma job can be daunting, but it doesn’t have to be. Here is a complete guide to the biopharma job hunt, from researching job openings to writing resumes and cover letters.
The new facility is expected to bolster innovation across all facets of manufacturing to allow the company to fully develop and commercialize cancer treatments for patients in need.
Pfizer, J&J and GSK indicate plans to file for regulatory approval by the end of this year for vaccines against RSV, which would see vaccines against the disease becoming available in 2023.
BioSpace looked at some of the efforts to improve the host system’s immune response, as well as others to improve allogeneic or autologous cell transplants.
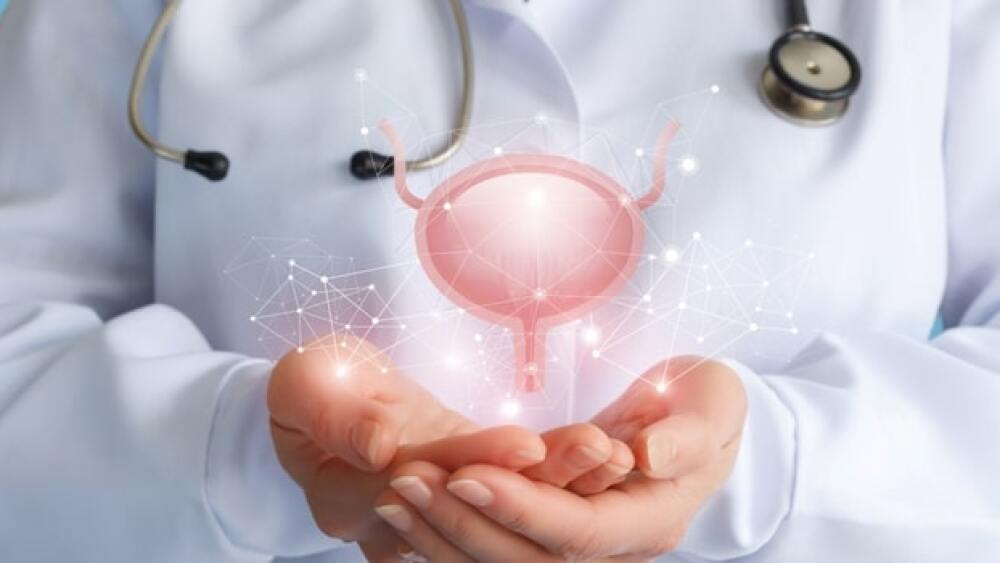
CG Oncology presented positive interim Phase II results for its Core1 trial studying the use of CG0070 at the American Association for Cancer Research (AACR) Annual Meeting.