All News
Wednesday, the FDA approved GSK’s daprodustat, now to be marketed as Jesduvroq, for the treatment of anemia due to chronic kidney disease in adult patients.
Karuna Therapeutics acquired exclusive rights to Goldfinch Bio’s investigational TRPC4/5 channel candidates in a deal potentially worth $520 million.
To help you take on any task you’re given at work, BioSpace has provided a guide detailing what to do when you’re asked to do things above your experience level.
Roche is dropping its investigational AKT inhibitor ipatasertib, which was being assessed in a Phase III trial for castration-resistant prostate cancer, as well as several other candidates.
Recent scientific papers report errors in CRISPR gene editing linked to ancestry.
Novartis is cleaning house, cutting its Huntington’s disease program along with several others. The company announced multiple program stops and delays in its full-year 2022 report Wednesday.
The partners will combine Miromatrix’s single-use bioengineered liver with Baxter’s PrisMax system, which is designed to provide individualized therapies to patients.
GSK has dropped two assets, an investigational celiac disease therapeutic and a Staphylococcus aureus vaccine hopeful, from its pipeline.
The FDA asked Taysha Gene Therapies to dose more patients in a double-blinded, placebo-controlled trial before submitting a BLA for its giant axonal neuropathy candidate.
As the 118th Congress kicks into gear, biopharma industry observers speculate that 2023 may be a challenging year.
Microbiologics, a global biotechnology products and services leader, announced today that Kristen Knox has been appointed as Chief Executive Officer (CEO) of the company.
Now more than ever, there is ample opportunity for life science candidates with only a bachelor’s degree. Still, there are certain things these candidates should know to ensure their success.
uniQure inked a deal with Apic Bio to gain development and commercialization rights to APB-102, a gene therapy for a rare, genetic form of ALS.
Pfizer reported full-year 2022 earnings Tuesday and provided 2023 projections far below the previous year’s totals.
The pharmaceutical industry is expected to spend more than $3 billion on artificial intelligence by 2025, but advocates say it is not yet living up to its potential.
Tuesday, de-extinction company Colossal Biosciences closed a mammoth, $150 million Series B financing round and announced plans to resurrect the iconic Dodo – extinct since the 17th century.
Vera Therapeutics released a 36-week per-protocol analysis of the Phase IIb trial assessing atacicept in IgA nephropathy.
Computer programming jobs in biopharma are on the rise, but candidates must have a specific skill set. To help, here are the best programming languages for those working in the life sciences.
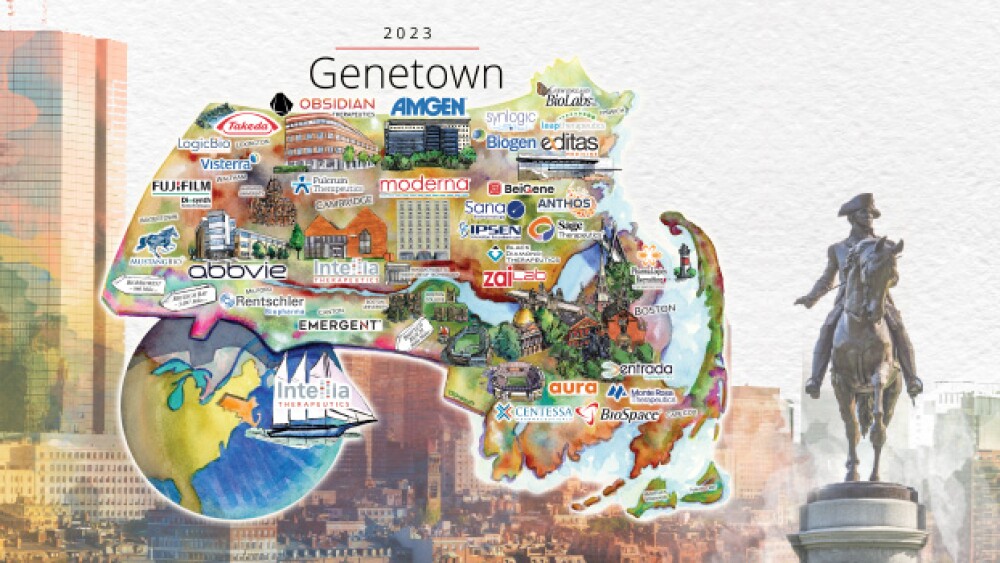
At a panel Friday, “Boston’s Booming Biotech System,” experts from industry and academia weighed in on the financial repercussions of 2022 and what it portends for 2023.
Amgen is implementing organizational changes including laying off approximately 300 team members, confirmed Monday.