Phase III
David S. Knopman and Joel S. Perlmutter both resigned from the committee after the approval of the drug aducanumab.
Pfizer says it expects the U.S. CDC’s ACI to meet in October to recommend the appropriate and safe use of pneumococcal vaccines, including PREVNAR 20, in adults.
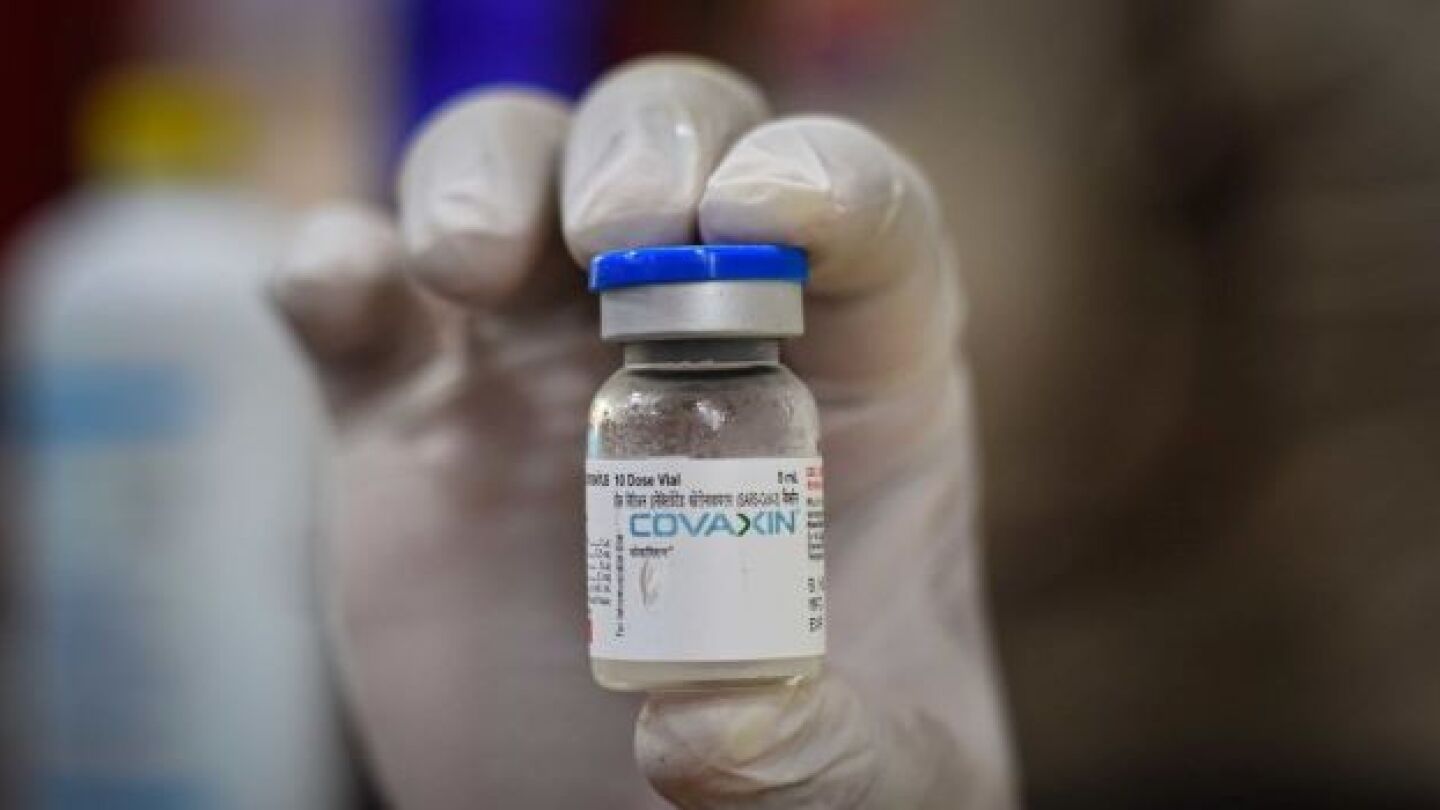
Bharat will conduct Phase IV studies to check real-world efficacy against the virus, while Ocugen says Covaxin will be a valuable tool in helping to end the COVID-19 pandemic.
Indivior PLC is collaborating with France-based Aelis Farma on the development of a treatment for cannabis use disorder and cannabis-induced psychosis.
The findings also highlight additional mechanistic, drug product stability, pharmacokinetics and toxicology studies that support the candidate as a potential HIV prevention and treatment.
Results from the Phase III TULIP® study show an antibody-drug conjugate from Byondis significantly prolonged PFS in patients with pretreated HER2-positive metastatic breast cancer.
Please check out the biopharma industry’s COVID-19 stories that are trending for June 8, 2021.
The tool developed by On Target boosts the ability of surgeons to find and remove cancerous lesions during surgical procedures, increasing progression free and overall survival.
Novartis flexed its clinical muscles over the weekend, announcing results from multiple studies including different types of cancer and kidney disease.
The U.S. Food and Drug Administration granted approval of Biogen’s aducanumab for the treatment of Alzheimer’s disease.
PRESS RELEASES