Rebiotix
NEWS
Ferring Pharmaceuticals today announced positive results across all five of its prospective trials for a live biotherapeutic designed to reduce recurrent C. difficile infection (rCDI).
The therapy is being developed to decrease C. difficile (C. diff) infection recurrences.
Biopharma companies strengthen their senior leadership teams and boards of directors with this week’s appointments.
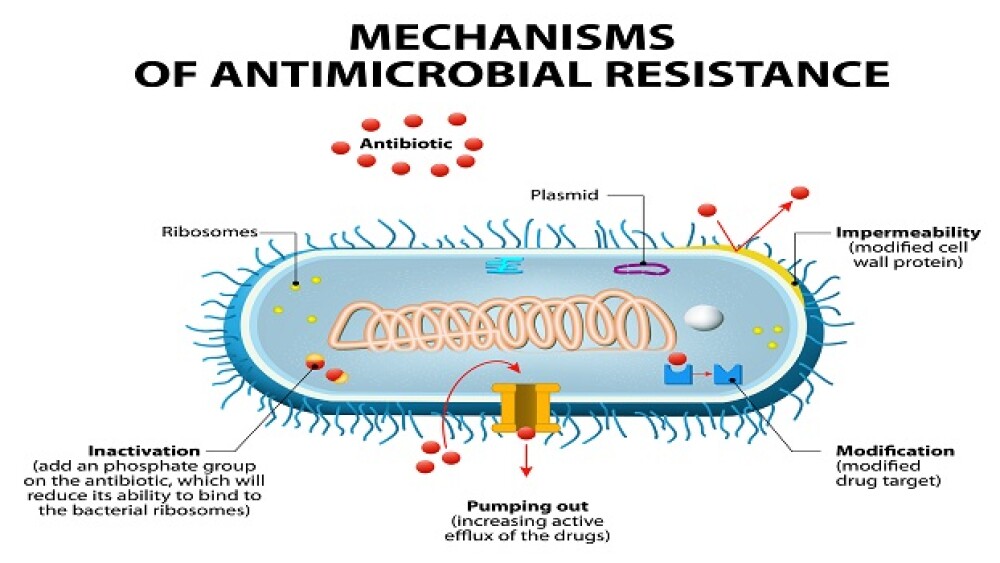
Arc Bio launched its proprietary antimicrobial resistance (AMR) software. It is the first in what it expects to be a series of next-generation sequencing (NGS)-based products for pathogen detection.
On May 29, the FDA cleared T2 Biosystems’ T2Bacteria Panel for diagnosis of sepsis. In that sepsis is the third-leading cause of death in the U.S., this emphasizes the importance of work in this area.
On May 1, the U.S. Food and Drug Administration (FDA) granted Scynexi’s oral formulation of SCY-078 to treat vulvovaginal candidiasis (VVC) and recurrent VVC both Qualified Infectious Diseases Product (QIDP) and Fast Track Designation.
Ferring Pharmaceuticals is buying Rebiotix. As part of the deal, will acquire Rebiotix’s lead program, RBX2660, a non-antibiotic treatment to prevent recurring Clostridium difficile infection (CDI).
JOBS
IN THE PRESS