Food and Drug Administration (FDA)
NEWS
On Wednesday, Swiss women’s health company ObsEva announced that it is dropping linzagolix, its leading candidate for uterine fibroids.
Ionis announced that the FDA has accepted its NDA for tofersen for SOD1-ALS. The application has been given the priority review designation and an action date of January 25, 2023.
The complaint alleges the agencies made an error under the Administrative Procedure Act, violating the Food Drug and Cosmetics Act by failing to approve Lumryz.
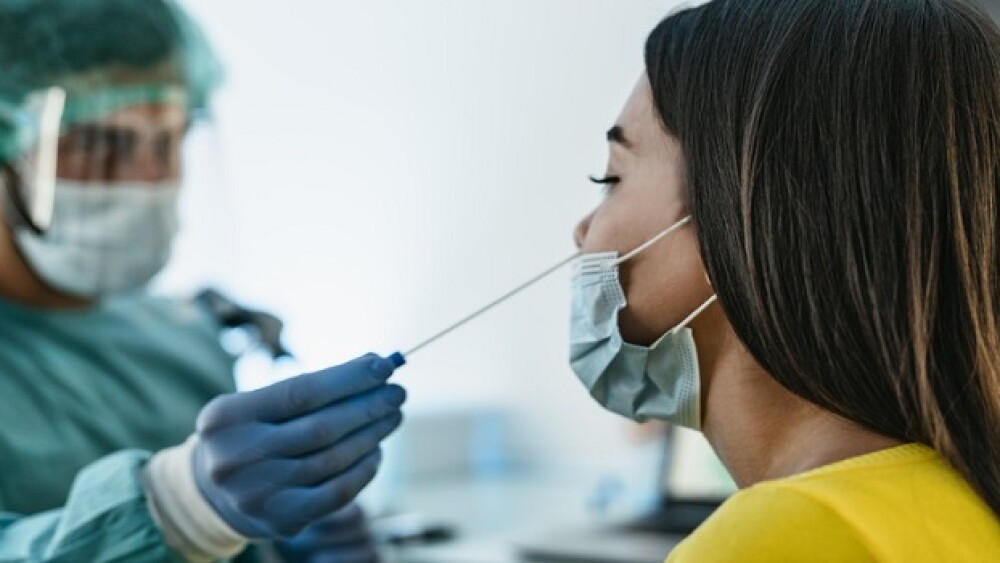
As the BA.4/BA.5 Omicron subvariants spread, researchers continue to develop COVID-19 therapies and approaches for preventing or treating the disease. For that and more, continue reading.
Mid-July is a relatively quiet time for the FDA in terms of drug approvals, but Incyte and Arcutis will have all eyes on the regulator as they await decisions in vitiligo and psoriasis.
Researchers point out that antibodies against amyloid are still considered an essential approach to treating Alzheimer’s, but the leading risk factor for sporadic AD is aging.
Omega Therapeutics announced Thursday that the Investigational New Drug (IND) application for its hepatocellular carcinoma (HCC) candidate OTX-2002 has been granted by the FDA.
The FDA granted Novavax’s COVID-19 vaccine Emergency Use Authorization after a lengthy and obstacle-riddled journey. It is authorized as a two-dose primary series for adults 18 years and older.
Gossamer Bio sold shares of its common stock to pave its runway through mid-2024 while Teva and AbbVie reach deals in opioid settlements.
JOBS
IN THE PRESS