Food and Drug Administration (FDA)
NEWS
The Trump administration has launched a sweeping federal review on the use of fetal tissue that includes the cancellation of a contract between a supplier and researchers at the U.S. Food and Drug Administration.
Politicians might deny climate change, but disease-carrying insects and their pathogens aren’t—they’re exploiting it.
Recently, BioSpace posted a question on its website, under the heading, “We Want to Hear from You! Top Life Sciences Trends.” Let’s take a look at the five trends survey participants mentioned.
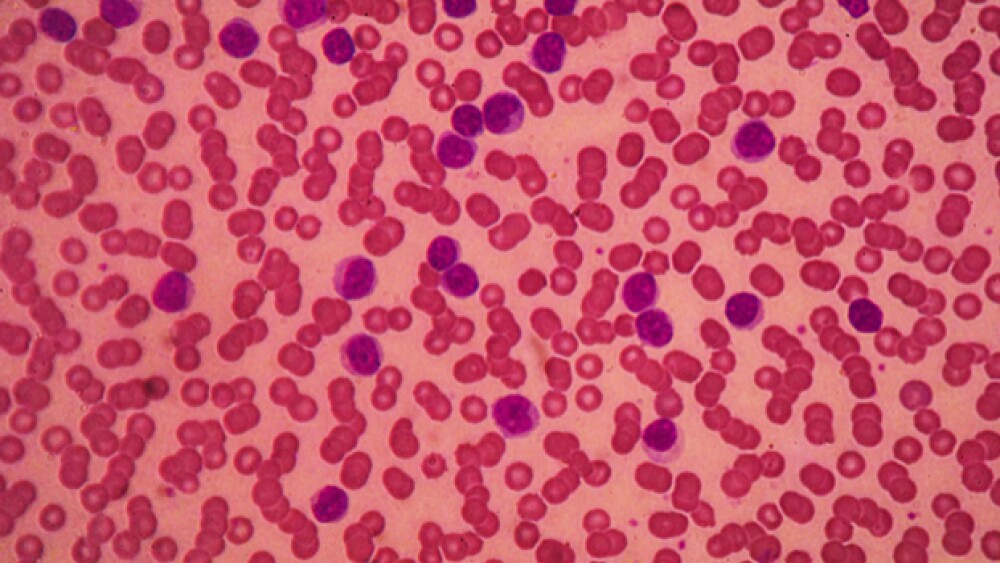
Shares of Verastem closed out Monday trading on a positive note and continues to climb in the premarket following the greenlight for leukemia drug Copiktra, an inhibitor of phosphoinositide 3-kinase (PI3K)-delta and PI3K-gamma.
Ovarian cancer is the fifth leading cause of cancer-related deaths in the United States. It is estimated that this year there will be more than 22,000 women in the U.S. diagnosed with the disease and about 14,000 disease-related deaths.
Akcea Therapeutics, based in Cambridge, Massachusetts, and Ionis Pharmaceuticals, headquartered in Carlsbad, California, released positive topline data from a Phase II trial of AKCEA-APO(a)-LRx in cardiovascular disease and lipoprotein(a).
Epizyme, an innovative epigenetic therapy development company, announced this morning that the U.S. Food and Drug Administration has removed their former partial clinical hold reported in April of this year.
The U.S. Food and Drug Administration (FDA) has a few target action dates scheduled for this week, including one for Sunday, September 23, which was approved in late August.
Shares of Acadia Pharmaceuticals soared more than 26 percent in trading on Thursday and continue to climb this morning in premarket trading after the U.S. Food and Drug Administration affirmed the safety profile of the company’s lead drug, Nuplazid.
JOBS
IN THE PRESS