Food and Drug Administration (FDA)
NEWS
The U.S. FDA granted Roche’s Venclexta (venetoclax) full approval in combination with azacytidine, or decitabine, or low-dose cytarabine for newly diagnosed acute myeloid leukemia in adults 75 years or older.
The U.S. Food and Drug Administration has several PDUFA dates for the rest of October, although two of them were already approved. Here’s a look.
Pfizer will not seek Emergency Use Authorization for its COVID-19 vaccine until the end of November even if the readout from a Phase III study expected later this month is positive.
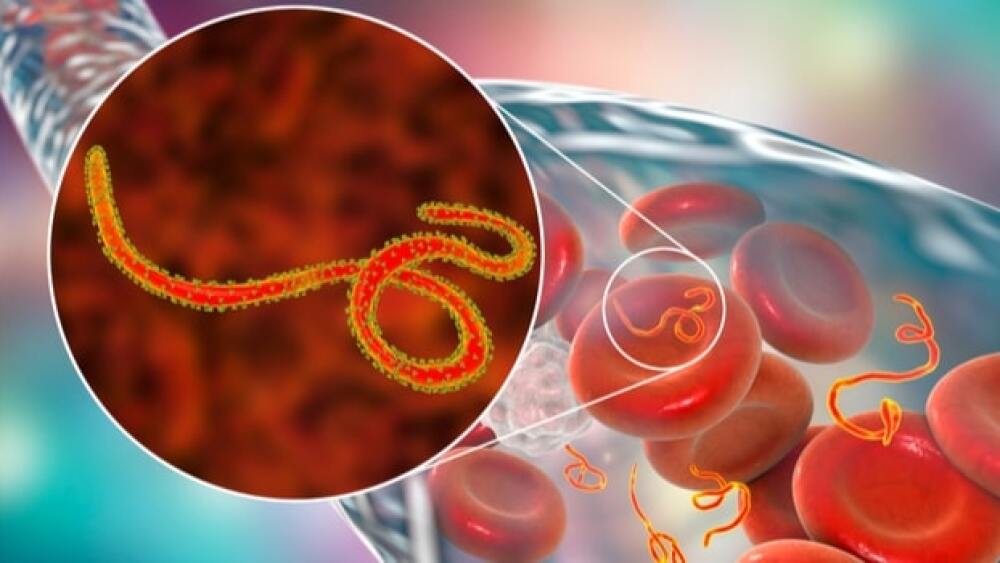
Regeneron announced that the U.S. Food and Drug Administration approved Inmazeb, a three-antibody cocktail to treat Ebola infections in adults and children.
To date, all the major clinical trials of vaccines against COVID-19 have been in adults.
Please check out the biopharma industry coronavirus (COVID-19) stories that are trending for October 13, 2020.
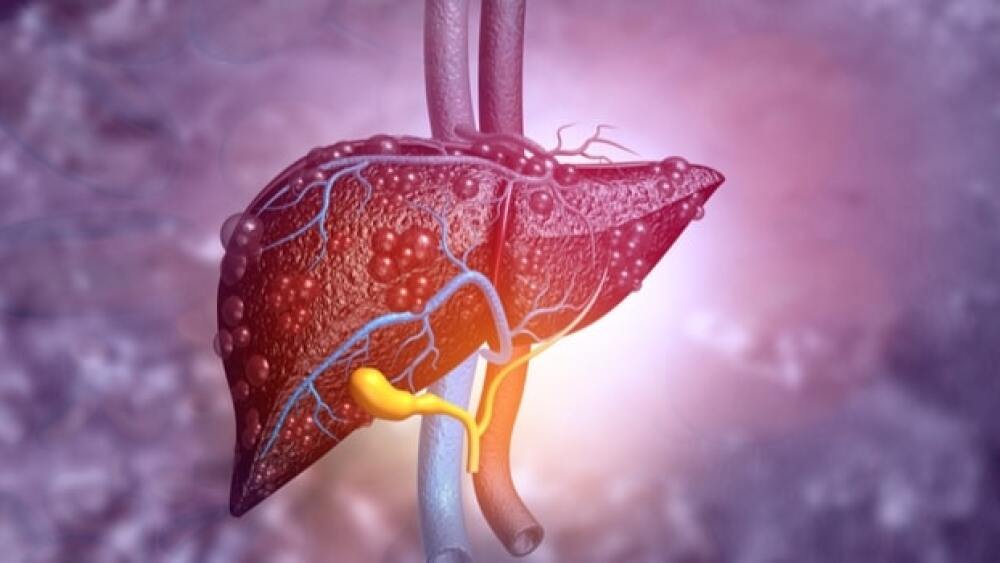
NASH is a metabolic disease similar to cirrhosis of the liver, but occurs in people who drink little, if any, alcoholic.
The FDA placed the hold onto the program hold pending the resolution of certain chemistry, manufacturing and controls (CMC) matters, Voyager said.
Although the Phase III trial for the Tramadol demonstrated statistically significant outcomes for all of the primary and many secondary endpoints, the FDA rejected the application.
JOBS
IN THE PRESS