Pfizer
NEWS
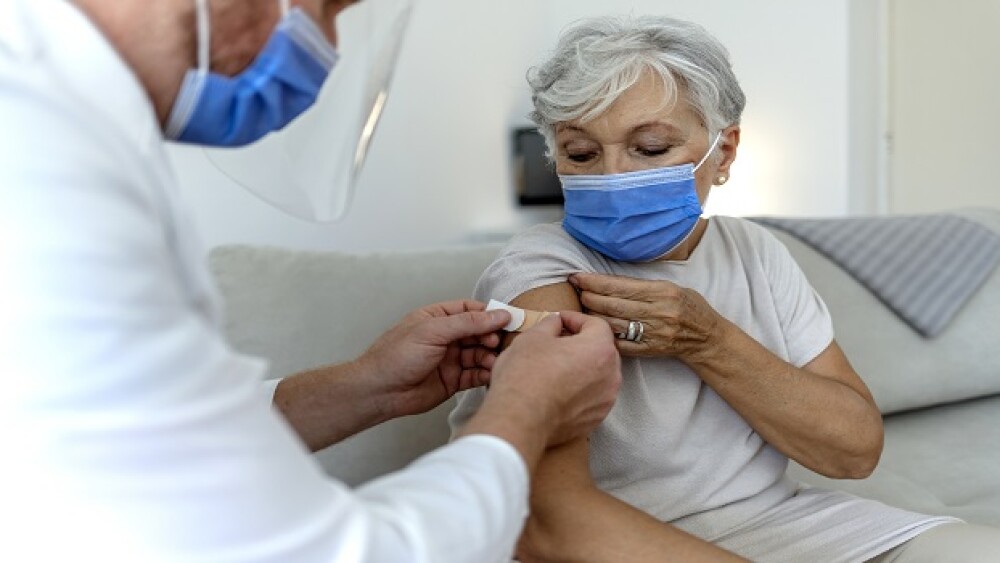
Without holding a meeting of its vaccines advisory committee, the U.S. Food and Drug Administration (FDA) authorized a fourth booster shot for everyone 50 years of age and older.
Pfizer stated that etrasimod patients achieved statistically significant improvements in primary endpoints of clinical remission and attained all secondary endpoints vs. placebo.
COVID-19 vaccines are the most-studied vaccines in history. This is partly why some data can appear so contradictory. For those and more COVID-19 stories, continue reading.
Although it was relatively quiet in COVID-19-related clinical trials, there was plenty of other clinical trial news. Here’s a look.
The study suggests that artificial sweeteners, which are readily available in coffee shops and used in diet soft drinks, increase the risks of breast cancer and obesity.
Pfizer has announced it received Breakthrough Therapy Designation from the FDA for its vaccine candidate intended to prevent infections caused by the respiratory syncytial virus (RSV).
Pfizer issued a statement on Monday regarding a voluntary recall of three of its products. The recall affects eleven lots of hypertension medications in total.
Pfizer is lining up a potential New Drug Application for an ulcerative colitis treatment for patients who are not seeing an improvement in their condition from currently available options.
Moderna reported positive interim results from the Phase II/III KidCOVE trial of its mRNA COVID-19 vaccine for children six months to under two years and two to six years of age.
JOBS
IN THE PRESS