All News
Despite the massive financing from venture capitalists that poured into Massachusetts in 2021, Bay State biopharma companies are struggling to find enough talent to fill the number of available jobs.
Altimmune announced Monday that the U.S. Food and Drug Administration (FDA) has approved a Phase II clinical trial for investigational new obesity drug pemvidutide.
The decision came after the Phase IIb study results demonstrating a statistically significant decrease in non-HDL-C and statistically significant reductions in triglycerides and ANGPTL3.
One week after Johnna Rossell departed Biogen for Enzyvant, Biogen announced two more members of its board are leaving.
WRNMC researchers have found a way to create a vaccine that can recognize multiple spike proteins at once by using ferritin. Here’s more about pan coronavirus vaccine.
The union has one primary goal – speed up discovery and development to get effective drugs to patients sooner.
News that a subvariant of the Omicron variant has appeared in the U.S. and parts of Europe doesn’t seem like good news. The latest data suggest it’s 1.5 times more transmissible than the original Omicron variant, but not necessarily deadlier.
Scientists believe that, perhaps, Alzheimer’s disease is a cause of not just beta-amyloid accumulation but, digging more deeply, immune system dysfunction.
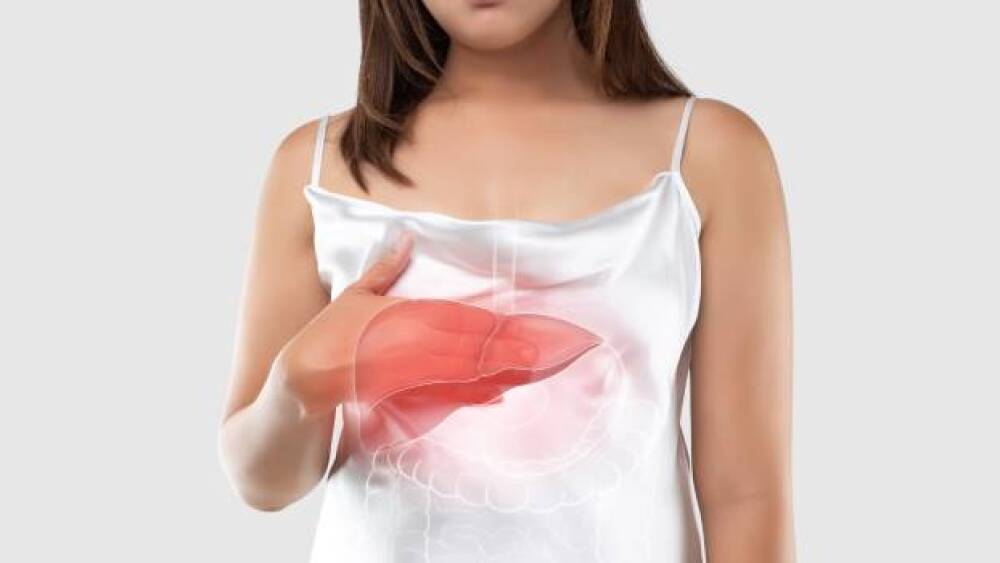
Shares of Madrigal Pharmaceuticals went up in premarket trading after the company announced positive topline data from its Phase III assessment of resmetirom in non-alcoholic fatty liver disease.
The use of body language during interview can ensure a good impression. Kinesics includes the use of posture, facial expression, movement, and gestures to communicate nonverbally.
The FDA’s decision is based on positive results from several Phase III studies, including the TENAYA and LUCERNE clinical trials.
What makes a candidate attractive? For Deschoolmeester, three critical elements stand out: pragmatism, creativity and collaboration.
Eli Lilly and Company announced that it was discontinuing the Phase III development program for Olumiant (baricitinib) in lupus.
TenSixteen Bio launched Thursday with a $40 million Series A financing round funded by Foresite Capital and GV, formerly Google Ventures.
The resubmission follows a Type B pre-BLA resubmission meeting with the US Food and Drug Administration (FDA).
Clinical trial news definitely picked up this final week of January. Here’s a look.
A new report showed the increased funding in 2021 mirrors national data that show accelerated financing in support of new therapies for multiple diseases, driven by COVID-19 concerns.
Researchers are expressing concern about a subvariant of Omicron dubbed BA.2 that appears to be tearing through Denmark. For that and more COVID-19 news, continue reading.
Oxford BioMedica and Homology Medicines have formed a symbiotic partnership to launch a full scope AAV manufacturing and innovation business.
Taysha said the data for TSHA-101 is the “first-ever to support the bicistronic vector approach in humans delivering both HEXA and HEXB genes in the endogenous ratio.”