All News
BridgeBio Pharma, Inc. (Nasdaq: BBIO) (BridgeBio), today announced positive interim results from PROPEL 2, a Phase 2 trial of the investigational therapy infigratinib in children with achondroplasia.
Researchers at the Institut Pasteur in Paris, France have found that the COVID-19 virus enters the brain through nanotubes to cause debilitating neurologic symptoms.
Martin Shkreli, commonly known as the “Pharma Bro,” announced Monday he has cofounded Druglike, a Web3 drug discovery software platform, despite a lifetime ban from the pharma industry.
Here’s a look at the latest Long COVID research and the reemergence of a therapy whose efficacy has yet to be proven.
Ionis announced that the FDA has accepted its NDA for tofersen for SOD1-ALS. The application has been given the priority review designation and an action date of January 25, 2023.
BridgeBio announces positive Phase II trial results for achondroplasia, Astellas-Seagen announces promising outcomes from Padcev Plus Keytruda Trial and Kelun-Biotech’s exclusive MSD deal.
The company is readjusting its strategy as it seeks ways to preserve share value. The goal to preserve as much as the $60 million it has in cash and cash equivalents as of the year’s second quarter.
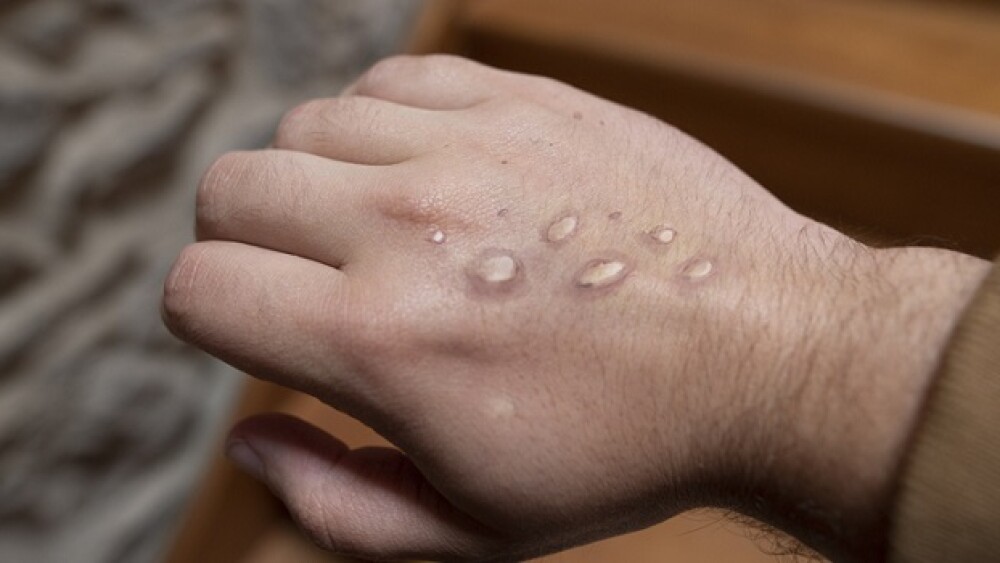
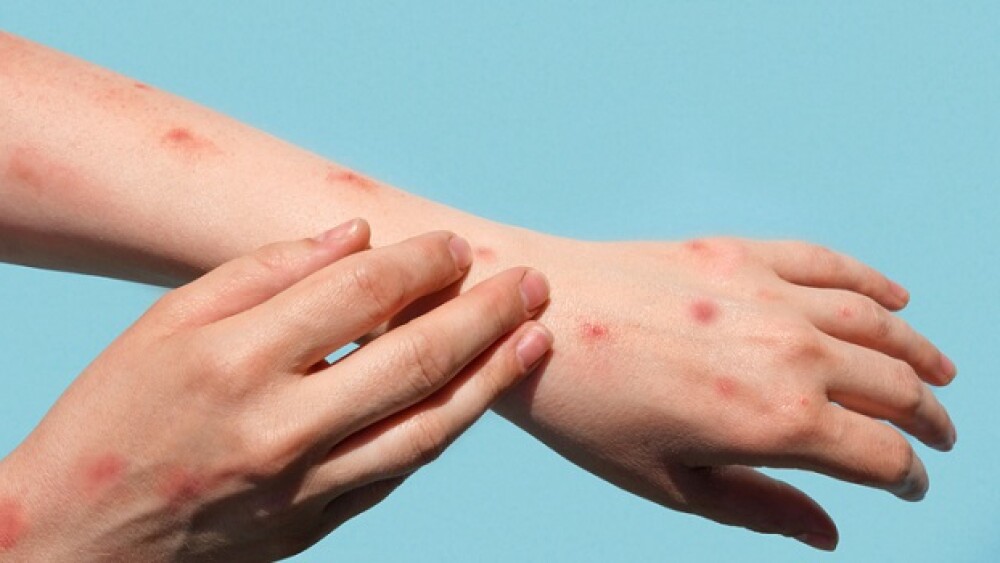
WHO declared the monkeypox outbreak a “public health emergency of international concern [PHEIC].” As COVID-19 wanes, Tonix, SIGA, Emergent Bio and others are now targeting monkeypox.
The U.S. Attorney’s Office was busy indicting nine people for charges related to insider trading. Some charges were unrelated, but three were specific to the biopharma industry.
The biotech industry is facing what some consider the worst times since its inception. Several market analysts recently shared their thoughts.
Here’s a look at global biopharma company news stories including Rona Therapeutics, Genuine Bio and NEOsphere Bio.
The transaction, Ginkgo’s largest acquisition to date, is expected to close by the first quarter of 2023, subject to regulatory approval and other closing conditions.
The complaint alleges the agencies made an error under the Administrative Procedure Act, violating the Food Drug and Cosmetics Act by failing to approve Lumryz.
Daiichi-Sankyo and AstraZeneca’s sBLA for HER2 directed Enhertu accepted by FDA. Novartis gets BLA acceptance for MS biosimilar. HOOKIPA’s IND for prostate cancer immunotherapy candidate accepted
A study published this month in Science discusses data that may support a devious alter ego of T-cells present within colorectal cancers that appear to inhibit and promote tumor progression.
The announcement signals that the WHO now views the outbreak as significant enough that a coordinated global response is necessary to control it.
Replay features next-generation genomic medicine technologies in its portfolio to address the needs of diseases that have large unmet needs.
In 2000, China did not have one company on the Forbes Global 2000 list. In 2021, it had 14. This is good for second place on this list, behind the United States which had 31.
FDA Weekly Review looks at the FDA’s actions related to drug approvals, IND approvals, designations and more. Here’s a look at what happened this week.
VistaGenTherapeutics announced data showing that its nasal spray candidate for anxiety, depression and other central nervous system disorders, fell short of its primary endpoint.