ImmunityBio, Inc
NEWS
ImmunityBio will part with 10 employees this quarter. Last fall, it cut 31 employees. The moves come as the biotech works to advance Anktiva in non-small cell lung cancer.
Madrigal Pharmaceuticals, X4 Pharmaceuticals and Day One Biopharmaceuticals secured their maiden approvals this year in metabolic dysfunction-associated steatohepatitis, WHIM syndrome and pediatric low-grade glioma. Geron Corporation and ImmunityBio also notched wins.
ImmunityBio will lay off 15 employees in California, mostly in El Segundo, effective Nov. 25. The company is also letting go 16 employees later this month.
For the first eight months of 2024, California had the most job postings live on BioSpace—37.7% more than Massachusetts. Last year, Massachusetts ranked No.1 for the same time period.
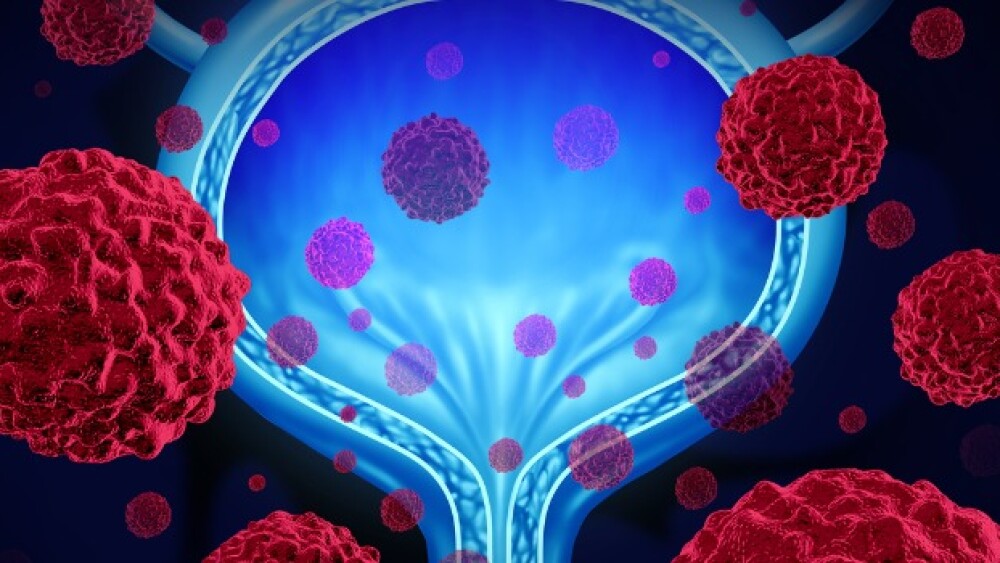
ImmunityBio will lay off 16 employees in California and said it expects to need more funding to commercialize Anktiva, approved in April for non-muscle invasive bladder cancer.
Bouncing back from a previous rejection, ImmunityBio on Monday secured the FDA’s green light for its IL-15 superagonist Anktiva for non-muscle invasive bladder cancer.
The FDA will close out April with five target action dates around indications that include pediatric seizures and a neurological cancer in children.
Trading cash for single-digit royalties on products, the deal provides ImmunityBio with the cash it’ll need to potentially commercialize its bladder cancer drug, if approved. The PDUFA date is April 23, 2024.
The regulator in a Complete Response Letter rejected ImmunityBio’s bladder cancer hopeful due to deficiencies with the company’s third-party contract manufacturer.
JOBS
IN THE PRESS