All News
This week, researchers published results from studies on treatments in lung disease, Alzheimer’s and various cancers. Here’s a look at that and more.
Life science companies made adjustments to their leadership teams with multiple appointments for chief commercial officers, chief business officers and more in this week’s Movers & Shakers.
Biohaven Pharma’s verdiperstat, an experimental treatment for amyotrophic lateral sclerosis, failed to distinguish itself from placebo in clinical testing, the company announced Thursday.
Two rare disease companies - Idera Pharmaceuticals and Aceragen - are merging to pursue the common goal of an FDA approval that could come as early as 2024.
Eisai and Biogen announced positive data from a Phase III trial in Alzheimer’s of its drug lecanemab. Still, questions remain about the drug’s implications for the future of Alzheimer’s treatment.
Novo Nordisk struck a licensing deal Thursday with Ventus Therapeutics valued at up to $700 million to develop and commercialize peripherally-restricted NLRP3 inhibitors.
The FDA has approved Regeneron and Sanofi’s Dupixent (dupilumab) for the treatment of adult patients with prurigo nodularis, making it the first drug approved for this indication.
New data from Axcella, PepGen, Dystrogen and Galecto are showing promise in challenging diseases, including NASH, Duchenne muscular dystrophy and myelofibrosis.
PhaseBio Pharmaceuticals reported its collaboration partner, SFJ Pharmaceuticals Group, informed the company it would need to return the rights to its lead program.
Ordaōs and NonExomics announced a research pact Wednesday to develop mini-proteins, called miniPROs, for three specific difficult-to-target rare cancers.
In honor of World Cancer Research Day, Sept. 24, BioSpace spoke with Elevation Oncology, Janssen, Merck and Teclison to learn more about the latest innovations in immuno-oncology.
A new formulation of NeuroSense’s amyotrophic lateral sclerosis therapeutic PrimeC could pave the way for an expansion of its ongoing Phase IIb PARADIGM study into the United States.
A cloudy ophthalmic space got a little clearer Wednesday with the two collaborations by Visus Therapeutics and Outlook Therapeutics, now preparing for FDA approval.
Genentech and privately-held Arsenal Biosciences forged a multi-year collaboration to identify critical success circuits in T cell-based therapies for solid tumors.
Pheon Therapeutics launched with $63 million in Series A financing and a vision of ushering in the next generation of antibody-drug conjugates.
TCR2 Therapeutics announced promising Phase I data for gavo-cel in mesothelin-expression that points toward effects in multiple solid tumors, especially ovarian cancer.
The anti-amyloid approach to treating Alzheimer’s disease is seeing new life after Eisai and Biogen announced that lecanemab slowed progression of disease in a Phase III study.
Funding initiatives this week saw money flow into cancer, rare liver diseases, respiratory depression, chemotherapy-related toxicities and a cutting-edge machine learning platform.
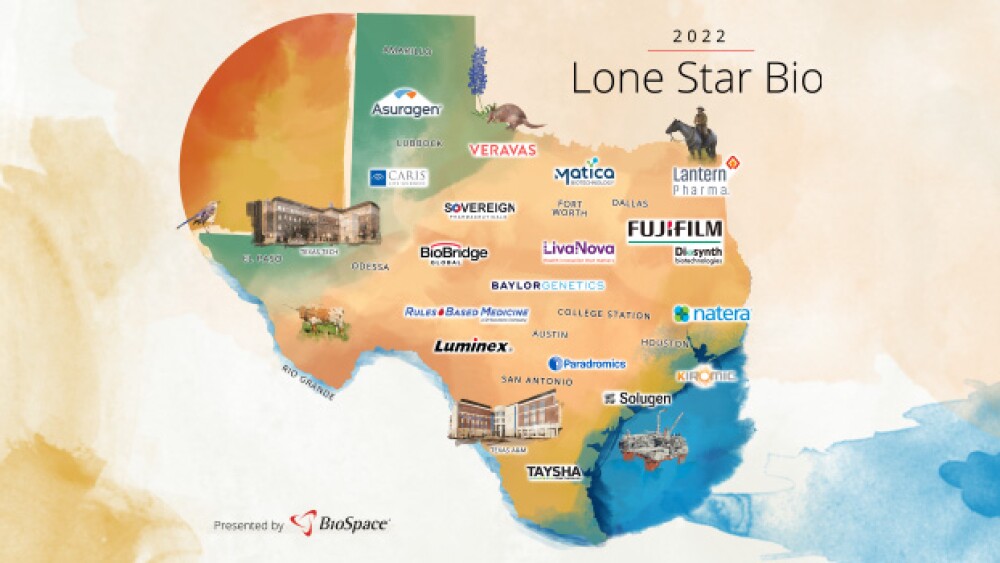
FUJIFILM Dyosynth Biotechnologies, Taysha Gene Therapies, Veravas highlight innovation stemming from the Lone Star state. BioSpace takes a deep dive into these and other Texas innovators.
Shares of Exicure, Inc. are falling after the company announced a strategic initiative that casts doubts on its future developmental programs.