All News
The World Muscle Society 2022 Congress is ongoing in Halifax, Canada, with numerous companies presenting cutting-edge research in neuromuscular diseases. Here’s a look.
Vividion Therapeutics and Tavros Therapeutics entered into a five-year collaboration agreement, valued at up to $930 million, to discover and develop four novel oncology targets.
Novartis’ gene therapy Zolgensma is again under a cloud of doubt after Nature Biotechnology retracted its 2010 article noting “issues” regarding data cited in a key figure in the report.
Merck and Moderna are moving forward with a personalized cancer vaccine as Merck exercised its option to develop and commercialize mRNA-4157/V940, the companies announced Wednesday.
This week, financing came in the form of loans, private placements and a Series B, throwing support behind an injectable heart failure drug, oral immunology therapies and an ASD treatment.
Nimbus Therapeutics scored a $496 million payday through a collaboration and licensing deal with pharma giant Eli Lilly for potential therapeutics for metabolic diseases.
In the wake of a global pandemic and economic downturn, the hiring market has turned on its head. BioSpace spoke with PharmaLogics Recruiting to learn how employers can stay competitive.
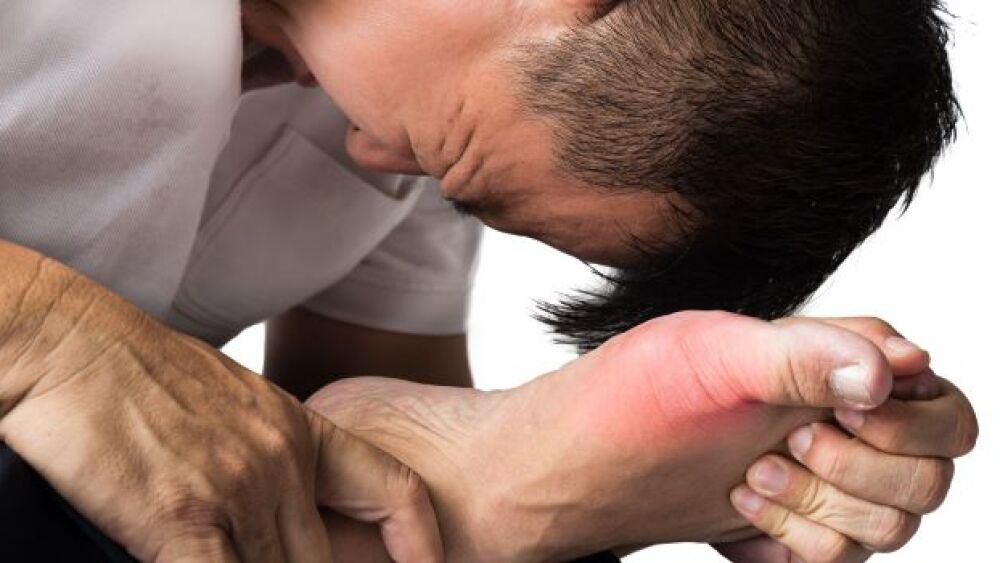
Incidence of gout - the most common inflammatory arthritis in the world - are on the rise. Arthrosi Therapeutics, Synlogic, Atom Bioscience and Ginkgo Bioworks are on the case with novel new approaches.
Forge Biologics’ novel gene therapy for Krabbe disease, a fatal neurodegenerative disorder, showed positive signals in a Phase I/II trial.
Albireo Pharma reported dazzling Phase III ASSERT data of Bylvay for Alagille syndrome (ALGS). The company is headed to U.S. and European regulators with expectations of regulatory filings in Q1 of 2023.
With $50 million in backing from Apple Tree Partners, Ascidian Therapeutics launches Tuesday with technology that allows for the treatment of some diseases through rewriting of RNA.
One year after raising $500 million, Neumora Therapeutics secured an additional $112 million in a Series B financing round to support the advancement of its neuroscience portfolio.
Biotechnology Innovation Organization, the world’s largest science and public advocacy organization, announced Monday it has tapped Rachel King to serve as interim President and CEO.
Last week, rumors spread that PureTech Health was preparing to merge with Nektar Therapeutics. Now the deal, never officially announced, has been called off.
ImmunityBio is laying off 38 employees at its Dunkirk site in New York, Amneal will shutter a Long Island facility and Rigel culls 30 employees following wAIHA regulatory decision.
Data from an ongoing Phase III trial showed Sanofi/Regeneron’s Dupixent can induce histological disease remission in young EoE patients aged 1–11.
Ginkgo Bioworks partnered with Merck on engineered enzymes for use as biocatalysts for Merck’s active pharmaceutical ingredient (API) manufacturing programs.
Immatics announced plans to raise about $110 million in a common stock offering following the release of promising early-stage data that supports its ACTengine technology platform.
On Friday, the FDA approved Boostrix, a vaccine administered during the third trimester of pregnancy to prevent pertussis in infants under 2 months of age.
SIFI, BeiGene and Novo Holdings shared promising updates on their proposed treatments for acanthamoeba keratitis, relapsed or refractory CCL and myasthenia gravis.