The 2025 BIO International Convention (BIO2025), the world’s largest annual biotech event, will take place from June 16 to 19 (U.S. time) at the Boston Convention & Exhibition Center. This year, Taipei City Government is partnering with six Taipei-based biotech companies, including PanCAD.ai Co., Ltd., Instant NanoBiosensors Co., Ltd., Bened Biomedical Co., Ltd., Cancer Free Biotech Ltd., Xantho Biotechnology Co., Ltd., and Energenesis Biomedical Co., Ltd., to showcase their innovations at the Taiwan Pavilion (Booth #1645) in the Taipei Exhibition Area.
Biotechnology is one of Taipei’s strategic industries. As the political, economic, and financial hub of Taiwan—as well as a center for science and innovation—Taipei is home to leading medical centers and top academic and research institutions, supported by a strong pool of biomedical talent. The city also benefits from a solid foundation for industry-academia-research collaboration. In addition, Taipei offers comprehensive infrastructure, a sound regulatory environment, and strong government support for the biotech and innovation sectors, creating fertile ground for biotech enterprises to grow. In the 2025 World’s Best Cities ranking, Taipei was named 7th in Asia, highlighting its strong international competitiveness and outstanding overall performance.
This year, six outstanding biotech companies were selected to represent Taipei at BIO 2025, spanning a wide range of fields including AI-powered early detection of pancreatic cancer, early diagnosis of Alzheimer’s disease, precision cancer medicine, plant-based novel drugs, psychobiotics, cancer organoids, and regenerative therapeutics. This diversity underscores the dynamic innovation capacity of Taipei’s biotech ecosystem. The following companies will be showcasing their innovations:
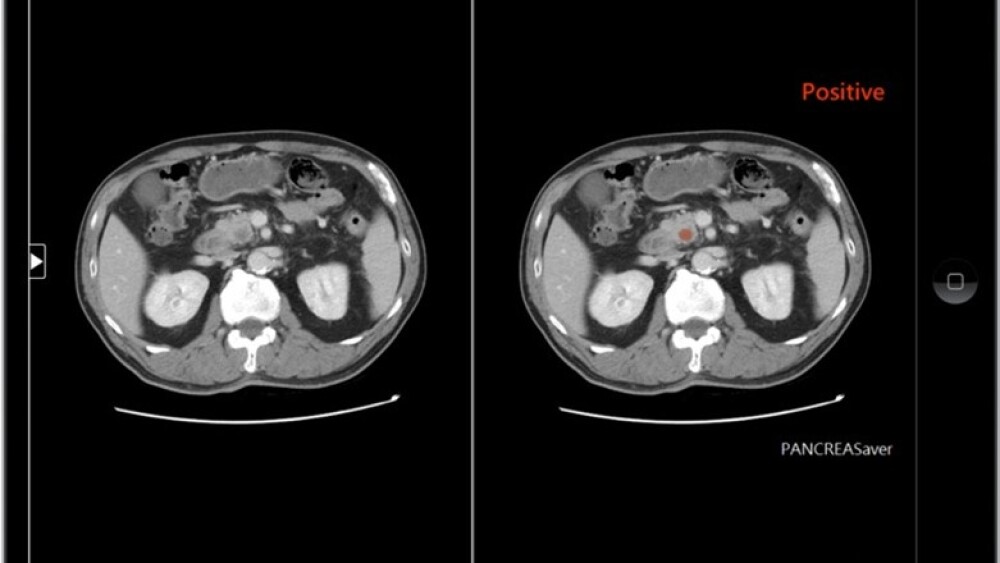
* PanCAD.ai developed “PANCREASaver,” the world’s first fully automated AI-powered pancreatic cancer detection system, targeting pancreatic cancer, one of the deadliest malignancies. The system autonomously identifies early-stage tumors on CT scans and pinpoints their locations, with an accuracy of 90%, as validated by using nationwide datasets. “PANCREASaver” has received regulatory approval as an innovative medical device from the Taiwan Food and Drug Administration (TFDA) as well as the US FDA Breakthrough Device Designation.
Website: PanCAD.ai
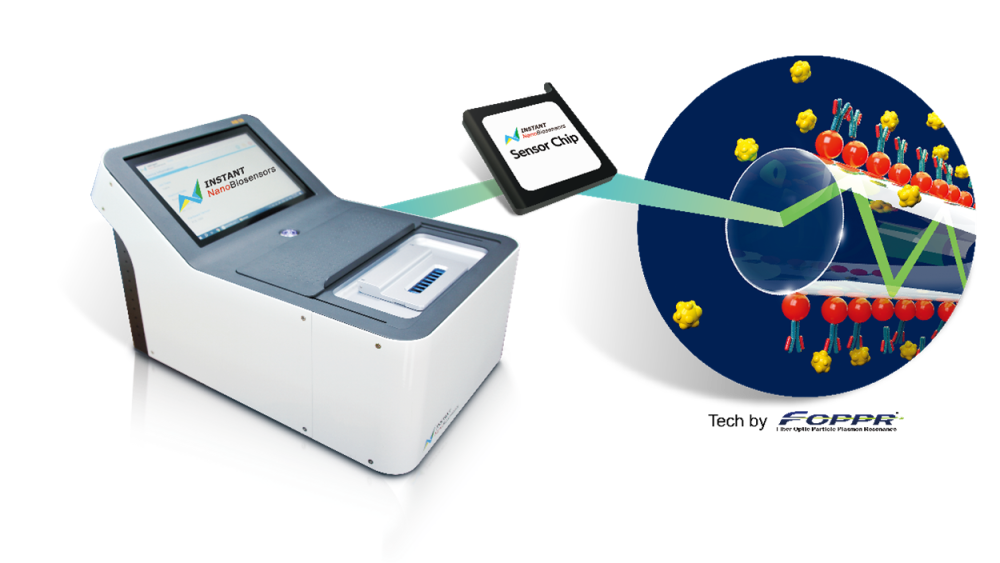
* Instant NanoBiosensors (INB) pioneers embedded precision medicine through its first-ever fiber optic particle plasmon resonance (FOPPR) and proprietary microfluidic chips, which empower non-invasive, rapid, ultra-sensitive, and cost-effective blood-testing platforms for the early detection and monitoring of Alzheimer’s disease. The platform is now used by the Amsterdam UMC and serves as a core diagnostic tool in the EU-funded Tau-ImMunE project. Beyond neurodegenerative diseases, INB also launched INLab NGS, a comprehensive precision medicine solution for leukemia that fully integrates software and hardware platforms to cover the entire workflow from sample processing to report generation. INLab NGS has been adopted by a leading U.S. medical institution and by CAP-accredited laboratories across major hospitals in Taiwan.
Website: Instant NanoBiosensors
*Bened Biomedical uses its proprietary psychobiotic strains—such as PS128, PS23, and PS150—for the prevention and management of neuropsychological disorders via the gut-brain axis. The company is actively conducting clinical trials in areas such as stress, anxiety, autism, and Parkinson’s disease. Bened is also behind the first medical probiotic in the U.S. designed specifically for the neurodiversity community. Recognized by multiple international market research firms, Bened is widely regarded as a leading innovator in the global psychobiotic and functional probiotic sectors.
Website: Bened Biomedical
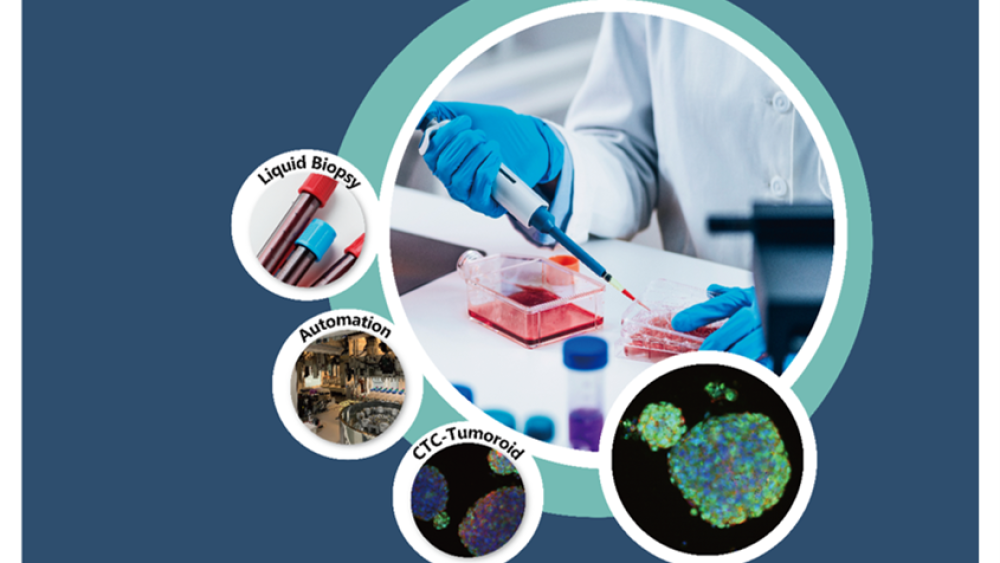
* CancerFree Biotech is dedicated to developing a proprietary 3D cancer cell culture system called Ex Vivo Avatar (E.V.A.). E.V.A. combines 3D cell culture scaffolding with AI image recognition technology. Using just 20 mL of patient blood, E.V.A. can promote tumor cell proliferation to generate heterogeneous organoid models that closely resemble the patient’s in vivo tumors. This platform may be extensively applied in two major areas: First, it can assist drug developers in validating the inhibitory effects of candidate drugs on real tumor cells, thus accelerating the screening of novel drugs. Second, it enables precision clinical medicine by allowing for multidrug sensitivity tests for individual patients, helping physicians to select the most promising treatment option.
Website: CancerFree Biotech
*Xantho Biotechnology is dedicated to developing safe and effective topical treatments for atopic dermatitis, offering patients a steroid-free option suitable for long-term use. Our lead product, GM-XANTHO, has successfully completed a multi-center, double-blind, placebo-controlled Phase IIa clinical trial, approved by both the U.S. FDA and TFDA. The clinical data analysis is finalized by Q2 2025. GM-XANTHO has demonstrated promising efficacy and safety profiles in clinical studies. We are actively seeking strategic partners to advance further development and commercialization, and we look forward to collaborating with pharmaceutical companies and investors to bring this innovative treatment to market.
Website: Xantho Biotechnology
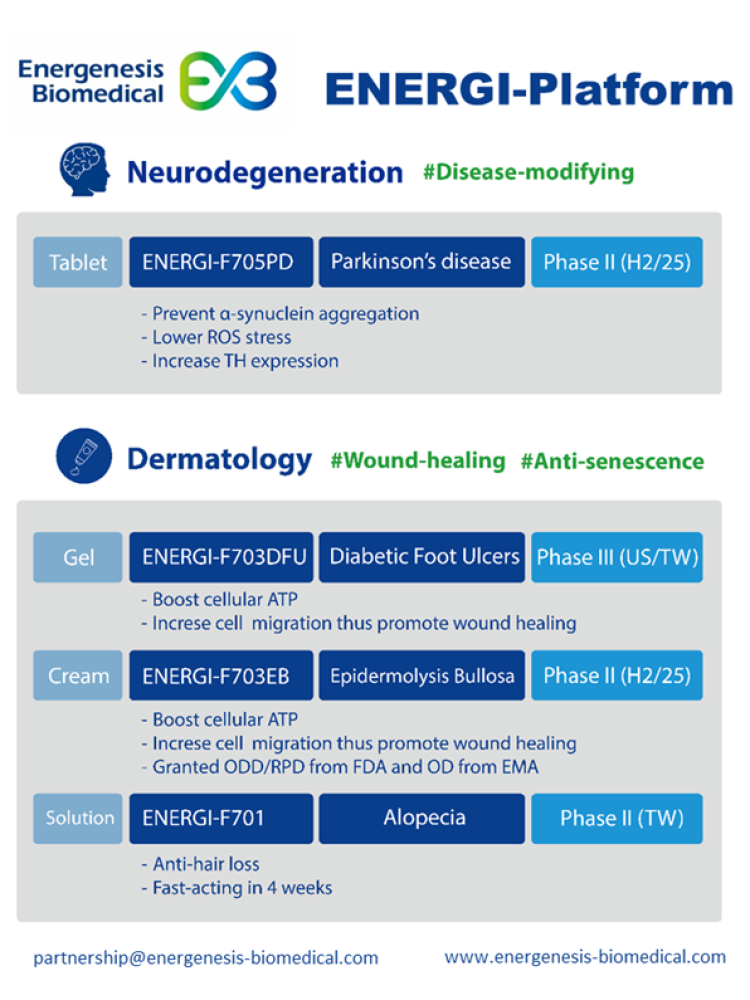
* Energenesis Biomedical uses its proprietary ENERGI platform to activate the self-healing mechanisms of cells to develop breakthrough therapies. The pipelines include ENERGI-F705PD, an innovative oral drug for treating Parkinson’s disease targeting anti-α-synuclein, which is currently undergoing a Phase I clinical trial and may advance to a Phase II trial after the results are obtained. The pipelines also include ENERGI-F703DFU gel, a Phase III project for treating diabetic foot ulcers, ENERGI-F701 topical solution, a Phase II project targeting hair loss, and ENERGI-F703EB cream, a project for treating epidermolysis bullosa (EB) which has received orphan drug designation (ODD) and rare pediatric disease (RPD) designation from the U.S. FDA, as well as orphan drug designation from the EMA and is currently advancing to Phase II trials. In addition, Energenesis is employing AI to explore drug repurposing opportunities to accelerate the development of new indications. Website: Energenesis Biomedical
The six outstanding biotech companies partnering with the Taipei City Government span a wide range of areas, including digital health, diagnostic testing, novel drug development, probiotics, and precision medicine, which fully demonstrate the capabilities of Taipei in biotech innovation and international commercialization. We sincerely invite industry leaders, investors, and media partners to visit the Taiwan Pavilion (Booth #1645) at BIO2025 to explore these innovative technologies and business opportunities. For more information, please visit the Bio@Taipei website.
Source: Department of Economic Development, Taipei City Government
Sponsored content is written and provided to BioSpace by the advertiser. It is published with the advertiser’s approval without contribution from BioSpace’s editorial and insights teams.