PQ Bypass Completes Enrollment in DETOUR2 Percutaneous Femoral-Popliteal Bypass Pivotal Study for Patients With Complex Peripheral Arterial Disease
FDA-designated Breakthrough Device now one step closer to PMA submission
MILPITAS, Calif.--(BUSINESS WIRE)-- PQ Bypass, an innovative medical device company pioneering advancements in the treatment of complex peripheral artery disease (PAD), announces enrollment of the final subject in the company’s flagship IDE, the DETOUR2 clinical trial. This important milestone occurs only a month after the Detour System entered the U.S. Food and Drug Administration’s Breakthrough Device Program.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20201006005791/en/
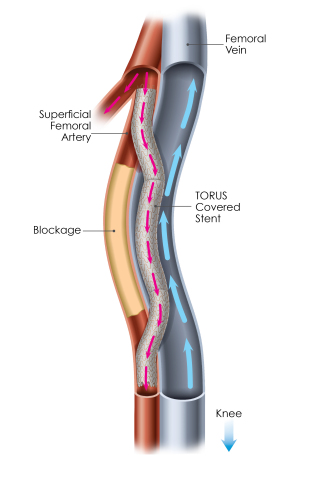
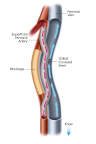
An image of PQ Bypass' completed Detour Procedure (Photo: Business Wire).
DETOUR2 is led by National Co-Principal Investigators Sean Lyden, MD, Professor and Chairman of the Department of Vascular Surgery at the Cleveland Clinic, and Jihad Mustapha, MD, President, CEO and Director of Endovascular Interventions at Advanced Cardiac and Vascular Centers. Both National Co-PI’s receive compensation for their duties in this role.
“The speed with which we were able to enroll DETOUR2 in 2020 speaks to the large patient population that exists with long-segment femoropopliteal disease that has sub-optimal endovascular options,” says Dr. Lyden.
“If percutaneous fem-pop bypass is shown to be safe and effective, similar to the outcomes demonstrated in DETOUR1 study, it could be a game changer for the way we treat complex, long-segment SFA disease today,” continues Dr. Mustapha.
DETOUR2 is a prospective, multicenter, trial evaluating the Detour System for percutaneous femoral-popliteal bypass in patients with extremely long, complex lesions in the SFA. The study enrolled 202 patients in 36 sites in the U.S. and Europe, and is assessing freedom from major adverse events (MAE) within 30 days of the index procedure as the primary safety endpoint. The primary effectiveness is primary patency at 12 months.
“After finishing enrollment in DETOUR2 and achieving the Breakthrough Device Designation, PQ Bypass is on track to deliver results from this study much earlier than what we originally expected,” says Heather Simonsen, President of PQ Bypass. “We would like to thank the DETOUR2 investigational sites for their ongoing contribution to this important research.”
“Reaching the end of enrollment in the DETOUR2 study during these trying times is yet another major milestone in a year filled with achievements for PQ Bypass,” adds Rich Ferrari, Chairman and CEO of PQ Bypass. “This groundbreaking technology is designed to change the treatment paradigm for complex SFA disease, much like what was observed when EVAR and TVAR were introduced to the field.”
About the Company:
PQ Bypass, Inc. is a rapidly advancing medical technology company pioneering a first-of-its-kind technology to address the complexity of treatment for severe peripheral arterial disease. Its proprietary Detour platform for percutaneous femoral-popliteal bypass–designated by the FDA as a Breakthrough Device–is designed to be a significant technological advancement enabling novel, transformational interventions in outpatient settings. PQ Bypass is a former Company-In-Residence at the Fogarty Institute for Innovation and is operated by recognized leaders in the medical device industry, including veterans from Boston Scientific, Phillips, Medtronic, Abbott and Johnson & Johnson.
PQ Bypass is currently sponsoring two multicenter IDE trials, DETOUR2 and TORUS2, focused on complex SFA disease.
For more information on DETOUR2, please visit https://clinicaltrials.gov/ct2/show/NCT03119233
For more information on TORUS2, please visit https://clinicaltrials.gov/ct2/show/NCT04130737
PQ Bypass is recognized by MedTech Outlook magazine as one of the Top 10 Cardiovascular Companies of 2019 and earned Frost and Sullivan’s European Technology Innovation award in 2017. The Detour System and the TORUS Stent Graft are limited by federal law to investigational use only and are not available for sale. For more information, please visit www.pqbypass.com
View source version on businesswire.com: https://www.businesswire.com/news/home/20201006005791/en/
Source: PQ Bypass