Neuronetrix, Inc. Announces Clinical Trial Collaboration With Premier Research Institutes University of Kentucky And Duke University Medical Center for a New Alzheimer's Disease Diagnostic Test
LOUISVILLE, KY--(Marketwire - July 02, 2010) - Researchers at the University of Kentucky Sanders-Brown Center on Aging and Duke University Medical Center have signed a contract with Neuronetrix, Inc. to kick-start a multi-center clinical trial to test brainwaves as useful cognitive biomarkers for Alzheimer's disease (AD).
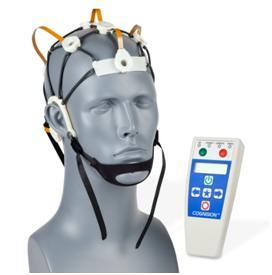
The COGNISION™ System, an innovative platform developed by Neuronetrix, enables objective assessment of cognitive function in a primary care setting using a technology called event-related potentials (ERPs). Previous scientific studies have demonstrated that ERPs can be used as a non-invasive tool for the early detection of AD. The new study is expected to validate these scientific studies in a real-world, multi-center environment.
Over the next 12 to 18 months, 200 volunteers will be recruited at four to six sites across the United States. Dr. Charles Smith, professor of neurology, and Dr. Gregory Jicha, assistant professor of neurology, UK College of Medicine, and researchers at the UK Sanders-Brown Center on Aging, will spearhead the effort. Dr. P. Murali Doraiswamy, professor of psychiatry, will lead the investigation at Duke University Medical Center. More centers will be added in the coming months.
ERPs will be measured from subjects' scalps while they listen to a series of auditory stimuli or "beeps." These ERPs will be used to train a computer to distinguish subjects with AD from healthy volunteers. This clinical trial builds on an earlier pilot study in which the portable COGNISION™ System was tested at the UK College of Medicine for data quality, ease-of-use, and patient tolerance.
"What sets our study apart from previous ERP studies in Alzheimer's patients is the rigorous clinical evaluation we will be performing on our subjects. This includes an extensive battery of advanced diagnostic tests to isolate Alzheimer's patients from those with other types of dementia. This is critical in achieving the high diagnostic accuracy we expect from our COGNISION™ System," says Mauktik Kulkarni, Director of Research and Clinical Affairs at Neuronetrix.
The COGNISION™ Test is expected to be the first objective cognitive biomarker to be approved for early detection of Alzheimer's disease. "The primary goal of our upcoming study is to replicate the high classification accuracy for Alzheimer's reported by Dr. Robi Polikar, one of our scientific advisors. We are looking forward to the study because the reported accuracy of ERPs is significantly higher than that observed in primary care centers. In addition, the secondary endpoints will give us clues about the utility of the COGNISION™ System in separating different types of dementias and monitoring the effects of experimental Alzheimer's drugs," says K.C. Fadem, COO of Neuronetrix.
About Alzheimer's Disease
Alzheimer's disease is a chronic neurodegenerative disease of the brain which eventually leads to death. Today, Alzheimer's disease affects over five million Americans with 500,000 new cases reported each year. The Centers for Disease Control recently reported that Alzheimer's disease is the seventh leading cause of death in the United States. Those affected by Alzheimer's disease survive only about half as long as those unaffected and of similar age. In 2005, Medicare/Medicaid spending totaled $112 billion on beneficiaries with Alzheimer's and other dementias.
About Neuronetrix
Neuronetrix is an emerging healthcare company focused on revolutionizing the treatment of patients with neurologic disorders by providing meaningful screening information to physicians early in the disease process. COGNISION™ is Neuronetrix's diagnostic technology developed to screen for these neurologic disorders.
Information about Neuronetrix is available at http://www.neuronetrix.com or by contacting K.C. Fadem at kfadem@neuronetrix.com or (502) 561-9040.
Contact:
K.C. Fadem
Neuronetrix, Inc.
1044 E. Chestnut St.
Louisville KY 40204
Ph: (502) 561-9040
Fax: (502) 561-9070
Email Contact
www.neuronetrix.com