Medizone Release: AsepticSure Hospital Disinfection System Achieves EPA Approval For US Market Entry
SAN FRANCISCO, Nov. 21, 2016 /PRNewswire/ -- Medizone International, Inc. (The Company) (OTCQB: MZEI) is pleased to announce that its unprecedented hospital room disinfection technology, AsepticSure®, has been given a green light for US market entry by the United States Environmental Protection Agency (EPA).
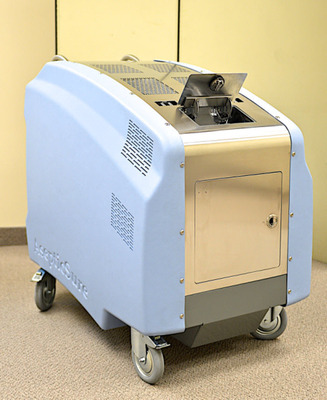
In August, Medizone announced that Cogmedix Inc., the Company's manufacturing partner, had obtained EPA company and establishment numbers to serve as a manufacturer for the AsepticSure System. Cogmedix already held FDA medical device manufacturing status. That EPA Registration allows Cogmedix to ship AsepticSure systems to hospitals and other users in the United States. Today, we are pleased to announce that EPA has granted a Registration number for the AsepticSure specific hydrogen peroxide catalyst used in the system. The AsepticSure Oxidative Catalyst is EPA approved for use in hospitals, clinics, food industry, sporting venues, and hotels to disinfect hard, non-porous surfaces at a 1.4% concentration.
Following the Christmas holiday period, Medizone anticipates entering into a brief trial/demonstration period in a major US hospital to confirm the disinfection results previously achieved in Canada are easily repeatable.
The Company is very optimistic that EPA approval for US market entry signals a turning point for our commercial development program as we move into 2017.
This Press Release may contain certain forward looking statements that could involve substantial risks and uncertainties, including, but not limited to, the results of ongoing clinical studies, economic conditions, product and technology development, production efficiencies, product demand, competitive products, competitive environment, successful testing and government regulatory issues. Additional risks are identified in the company's filings made with the Securities and Exchange Commission.
For press information on Medizone International, please contact:
John Pentony, Investor and Media Relations
Medizone International, Inc.
T: 01 415 331-0202
E: j.pentony@medizoneint.com
For more information, visit:
http://www.medizoneint.com
Email: operations@medizoneint.com
Photo - http://photos.prnewswire.com/prnh/20161118/441206
To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/asepticsure-hospital-disinfection-system-achieves-epa-approval-for-us-market-entry-300366081.html
SOURCE Medizone International, Inc.

