Follica Announces Positive Feedback From End of Phase 2 Meeting With FDA for Its Lead Program to Treat Male Androgenetic Alopecia
Company plans to initiate its Phase 3 program this year
BOSTON--(BUSINESS WIRE)-- Follica, Inc. (“Follica”), a biotechnology company developing a regenerative platform designed to treat androgenetic alopecia, epithelial aging and other related conditions, today announced positive feedback from a meeting with the U.S. Food and Drug Administration (FDA) as the company prepares to advance its lead program into Phase 3 development following a successful safety and efficacy optimization study for the treatment of hair loss in male androgenetic alopecia announced in December 2019.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20200603005934/en/
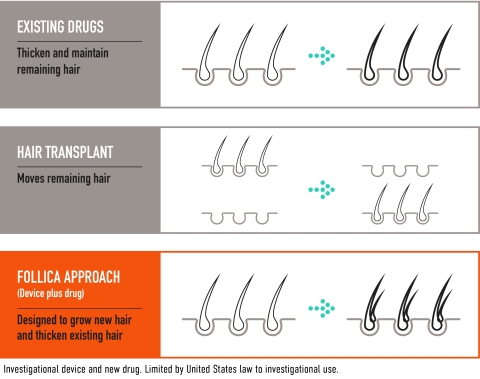
Follica’s approach, which is designed to stimulate the growth of new follicles and new hair, is being developed as a potential new option for the millions of people seeking treatments to grow new hair. (Graphic: Business Wire)
Follica plans to launch its Phase 3 program this year. Overall, approximately 280 patients will be enrolled, with efficacy assessed against two co-primary endpoints: visible (non-vellus) hair count and patient-reported outcomes on a pre-established scale. The randomized, controlled, double-blinded studies will be conducted in multiple centers across the U.S. A maximal use study to further understand the pharmacokinetics of the treatment will be conducted in parallel. The trial design is consistent with feedback from the FDA during the End of Phase 2 meeting.
“In the U.S. alone, 47 million men are affected by progressive hair loss caused by androgenetic alopecia, a condition that is largely unresolved today, leaving many dissatisfied with the current available treatments and looking for a new alternative. Our recent safety and optimization study points to a new level of effect, enabled by our proprietary approach, which stimulates the growth of new follicles and new hair,” said Jason Bhardwaj, chief executive officer of Follica. “We’re grateful to the FDA for their guidance as we prepare for our pivotal program, and we look forward to advancing the development of our treatment regimen, which has demonstrated strong potential to address the current need for those who seek treatment for androgenetic alopecia.”
Follica’s approach is based on generating an “embryonic window” in adult scalp cells via a series of short office-based treatments with its proprietary Hair Follicle Neogenesis (HFN) device. The scalp treatments, which last just a few minutes, stimulate stem cells and enable the growth of new hair follicles. A topical drug is then applied to enhance efficacy by growing and thickening new hair follicles and hair on the scalp.
Follica reported topline results from its safety and optimization study in December 2019. That trial was designed to select the optimal treatment regimen using Follica’s proprietary HFN device in combination with a topical drug and successfully met its primary endpoint. The selected treatment regimen demonstrated a statistically significant 44% improvement of visible (non-vellus) hair count after three months of treatment compared to baseline (p < 0.001, n = 19). Across all three treatment arms, the overall improvement of visible (non-vellus) hair count after three months of treatment was 29% compared to baseline (p < 0.001, n = 48), reflecting a clinical benefit across the entire trial population and a substantially improved outcome with the optimal treatment regimen. Additionally, a prespecified analysis comparing the 44% change in visible (non-vellus) hair count to a 12% historical benchmark set by approved pharmaceutical products established statistical significance (p = 0.005).
In addition to the safety and optimization study, Follica has validated its approach in prior clinical studies using prototype HFN devices with different treatment parameters and therapeutic compounds. Follica’s translational work builds on research by George Cotsarelis, M.D., who isolated and characterized the expression pattern of stem cells from a critical region of the follicle. An expert in epithelial stem cell biology, Dr. Cotsarelis is chair of the department of dermatology at the University of Pennsylvania and a co-founder of Follica.
About Androgenetic Alopecia
Androgenetic alopecia represents the most common form of hair loss in men and women, with an estimated 90 million people who are eligible for treatment in the United States alone. Only two drugs, both of which have demonstrated a 12% increase of non-vellus hair count over baseline for their primary endpoints, are currently approved for the treatment of androgenetic alopecia1. The most effective current approach for the treatment of hair loss is hair transplant surgery, comprising a range of invasive, expensive procedures for a subset of patients who have enough donor hair to be eligible. As a result, there remains a significant need for safe, effective, non-surgical treatments to grow new hair.
About Follica
Follica is a biotechnology company developing a regenerative platform designed to treat androgenetic alopecia, epithelial aging and other related conditions. Founded by PureTech (LSE:PRTC), a co-inventor of the current platform, and a group of world-renowned experts in hair follicle biology and regenerative medicine, Follica’s experimental treatment platform has been shown to stimulate the development of new hair follicles and hair in three previously conducted clinical studies. The company’s proprietary treatment is designed to induce an embryonic window via a device with optimized parameters to initiate hair follicle neogenesis, the formation of new hair follicles from epithelial (skin) stem cells. This process is enhanced through the application of a topical compound. Follica completed a safety and efficacy optimization study in 2019, and its Phase 3 program in male androgenetic alopecia is expected to begin in 2020. Follica’s technology is based on work originating from the University of Pennsylvania that has been further developed by Follica’s internal program. Follica’s extensive IP portfolio includes IP exclusively licensed from the University of Pennsylvania as well as Follica-owned IP.
1 Olsen EA et al, J Am Acad Dermatol. 2002 Sep;47(3):377-85
Olsen EA et al, J Am Acad Dermatol. 2007 Nov;57(5):767-74. Epub 2007 Aug 29
Price VH et al, J Am Acad Dermatol. 2002 Apr;46(4):517-23
Kaufman et al, J Am Acad Dermatol. 1998 Oct; 39(4):578-589
View source version on businesswire.com: https://www.businesswire.com/news/home/20200603005934/en/
Investors
Allison Mead Talbot
+1 617 651 3156
amt@puretechhealth.com
U.S. media
Stephanie Simon
+1 617 581 9333
stephanie@tenbridgecommunications.com
Source: Follica, Inc.
Follica’s approach, which is designed to stimulate the growth of new follicles and new hair, is being developed as a potential new option for the millions of people seeking treatments to grow new hair. (Graphic: Business Wire)
View this news release and multimedia online at:
http://www.businesswire.com/news/home/20200603005934/en



