Evelo Biosciences Announces Highly Significant Reductions in Clinically Validated Inflammatory Cytokines in EDP1815 Phase 2 Psoriasis Trial
- Treatment with EDP1815 led to reduced production of cytokines IL-6, IL-8, and TNF in blood cells
- Cytokine analysis supports gut-restricted action of EDP1815 to resolve inflammation throughout the body
- Reductions in IL-12b, IL-17, and IL-23 in skin lesions - all targets of approved biologics for psoriasis
- Support for broad potential of SINTAX medicines and EDP1815 in psoriasis and atopic dermatitis
CAMBRIDGE, Mass., Feb. 07, 2022 (GLOBE NEWSWIRE) -- Evelo Biosciences Inc. (Nasdaq:EVLO), a clinical stage biotechnology company developing small intestinal axis (SINTAX™) medicines as a new modality of orally delivered treatments for inflammatory diseases, today announced the results of immunological biomarker analyses from its previously reported Phase 2 trial of orally-dosed EDP1815 in mild and moderate psoriasis.
Evelo previously reported reductions in inflammatory cytokines in a Phase 1b trial of EDP1815 in mild and moderate psoriasis. These data have now been replicated in the Phase 2 psoriasis trial, with high statistical significance.
Blood samples were taken from 96 patients at baseline and after 16 weeks of dosing with EDP1815 or placebo. The figure below shows the changes in pro-inflammatory cytokines interleukin 6 (IL-6), interleukin 8 (IL-8) and tumor necrosis factor (TNF). Each vertical bar represents the fold change up or down from 0 in ex vivo stimulated cytokine production between the baseline and week 16 samples from a patient. Three different stimuli were used on each sample and the results from all three stimuli are presented together in the figures, giving the aggregate N (sample) numbers shown in the figures.
Treatment with EDP1815 led to a statistically significant reduction in the release of cytokines compared to placebo: IL-6 (p=0.0003), IL-8 (p=0.0007), and TNF (p=0.0037). The effect of EDP1815 is clearly seen by the deep tail of reduced cytokine production on the left of the distribution for each cytokine, which is absent in the placebo groups. There was no worsening compared to placebo on the right of the distributions, resulting in the overall significant difference between EDP1815 and placebo.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/17cbd920-fd68-4333-bee4-7566a3005f59
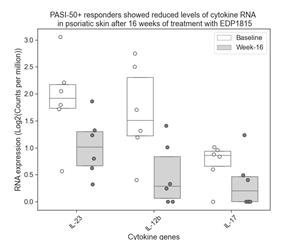
In addition, skin biopsies of active lesions were taken from a subset of six patients who received EDP1815 and achieved at least a 50% improvement in their Psoriasis Area and Severity Index (PASI) score (PASI-50) from baseline at week 16. RNAseq analysis showed reductions in transcript levels for psoriasis-relevant cytokines interleukin 23 (IL-23), interleukin 12b (IL-12b), and interleukin 17 (IL-17) in these lesions between baseline and week 16. The box plot below shows the median and interquartile ranges, as well as individual values of the cytokine expression levels in the skin, at baseline and week 16. These data were consistent with the systemic effects in blood described above, suggesting that EDP1815 reduces inflammation in the skin by modulating multiple proinflammatory cytokines systemically.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/ab175bd5-8a5f-45bb-82ba-65593ea47843
These data highlight the biology of SINTAX and support the development of a new class of medicine that acts locally in the small intestine to exert significant effects on inflammation throughout the body. There was no observed distribution of EDP1815 outside the gut. In the Phase 2 trial, EDP1815 was observed to have safety and tolerability data comparable to placebo. Six-month follow-up efficacy and safety data from the Phase 2 trial will be available later this quarter.
"Evelo has uncovered a central governing system of human biology - the small intestinal axis - opening up the possibility of a new type of safe and well-tolerated medicine with the potential to transform the treatment of all stages of inflammatory diseases globally,” said Simba Gill, Ph.D., Chief Executive Officer of Evelo. “Our accumulating clinical results, today’s data showing mechanisms of inflammation resolution in patients, and the safety and tolerability results observed with EDP1815, combine for an attractive probability of success for EDP1815 and Evelo’s pipeline of SINTAX medicines. These data provide the clearest scientific evidence yet for the potential of EDP1815 in psoriasis, atopic dermatitis, and beyond, and show that targeting SINTAX can lead to broad inflammation resolution. We are advancing EDP1815 towards Phase 3 clinical trials in psoriasis and expect to start dosing patients in the Phase 2 clinical trial in atopic dermatitis soon. We also recently published peer-reviewed preclinical data on a SINTAX development candidate, detailing its cellular mechanism of action which induced systemic anti-inflammatory effects while remaining gut-restricted."
About the Phase 2 Ex vivo Stimulation Analysis Protocol
Blood samples were taken from 96 patients at baseline and after 16 weeks of daily oral administration of EDP1815 (N=74) or placebo (N=22) in Evelo’s Phase 2 clinical trial in mild and moderate psoriasis. Whole blood was incubated with three stimuli separately: lipopolysaccharide (LPS), antibodies to CD3 and CD28, and staphylococcal enterotoxin B (SEB), which cover a broad range of immune cell function. Analyses were performed on blood samples from 96 of the patients in the trial. Fifty-five of these patients achieved at least a PASI-50 at week 16 or had PASI scores greater than 150% worse than baseline at week 16, selected from both active and placebo treatment groups. The other 41 patients were drawn at random from active and placebo groups pro rata to assemble a representative sample of the trial population, enriched by both tails of PASI outcomes. The differences in ex vivo stimulated cytokine production at baseline and week 16 were calculated.
About EDP1815
EDP1815 is an investigational oral medicine being developed for the treatment of inflammatory diseases. It is a non-live pharmaceutical preparation of a strain of Prevotella histicola, selected for its potential to provide systemic pharmacological effects after oral administration with gut-restricted distribution. Being non-live, it has not been observed to colonize the gut or modify the microbiome. Preclinically, EDP1815 had anti-inflammatory effects in models that cover multiple pathways of inflammation, Th1, Th2, and Th17. Clinical results from multiple independent cohorts provide evidence supporting EDP1815’s potential to address Th1, Th2 and Th17-mediated inflammation.
About EDP1815-201
EDP1815-201 is a double-blind, placebo-controlled, dose-ranging Phase 2 trial designed to evaluate three doses of an enteric capsule formulation of EDP1815 versus placebo in 249 patients with mild and moderate psoriasis over a 16-week treatment period. In the trial, the PASI scores were assessed by both mean changes from baseline and responder rates. The primary endpoint is mean percentage reduction in PASI score at 16 weeks. Secondary endpoints include the proportion of trial participants who achieve a PASI-50 response or greater and other clinical measures of disease such as Physicians Global Assessment (PGA), Body Surface Area (BSA), PGA x BSA, Psoriasis Severity Index (PSI), and Dermatology Life Quality Index (DLQI). The initial treatment phase of the trial is complete. A six-month follow-up phase of the trial is ongoing.
About Psoriasis
Psoriasis is a common chronic immune-mediated inflammatory skin disease, affecting up to 3% of the population worldwide. The disease is driven by Th17-inflammation, which results in the formation of thick red plaques with scaling. Psoriatic lesions can appear anywhere on the body but are most often seen on the knees, elbows, scalp, and lumbar area. In addition to the skin lesions, there are systemic manifestations of the disease including arthritis and fatigue, and a strong association with depression and metabolic syndrome.
Patients with mild and moderate psoriasis are underserved by current treatments. Topical therapies do not control systemic inflammation, have low rates of compliance, and in the case of topical steroids are not recommended for long-term use. The majority of novel therapies, including injectable high-cost biologics, are only approved for patients with moderate and severe disease. Even in the severe patient population, the majority of eligible patients do not receive biologics, instead opting for topical therapies or oral systemic therapies, which are associated with tolerability issues and/or with monitoring requirements tied to safety concerns.
About Evelo Biosciences
Evelo Biosciences is a clinical stage biotechnology company developing orally delivered product candidates that are designed to act on the small intestinal axis, SINTAX™, with systemic therapeutic effects. SINTAX plays a central role in governing the immune, metabolic, and neurological systems. The Company’s first product candidates are pharmaceutical preparations of single strains of microbes selected for their potential to offer defined pharmacological properties. Evelo’s therapies have the potential to be effective, safe, and affordable medicines to improve the lives of people with a broad range of inflammatory dieases.
Evelo currently has three product candidates in development: EDP1815, EDP1867, and EDP2939 for the treatment of inflammatory diseases. Evelo is advancing additional product candidates in other disease areas.
For more information, please visit www.evelobio.com and engage with Evelo on LinkedIn.
Forward Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this press release that do not relate to matters of historical fact should be considered forward-looking statements, including statements concerning the development of EDP1815 and our other product candidates, and the promise and potential impact of our product candidates.
These forward-looking statements are based on management's current expectations. These statements are neither promises nor guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, but not limited to, the following: the impact of the COVID-19 pandemic on our operations, including our preclinical studies and clinical trials, and the continuity of our business; we have incurred significant losses, are not currently profitable and may never become profitable; our need for additional funding; our limited operating history; our unproven approach to therapeutic intervention; the lengthy, expensive, and uncertain process of clinical drug development, including potential delays in regulatory approval; our reliance on third parties and collaborators to expand our microbial library, conduct our clinical trials, manufacture our product candidates, and develop and commercialize our product candidates, if approved; our lack of experience in manufacturing, selling, marketing, and distributing our product candidates; failure to compete successfully against other drug companies; protection of our proprietary technology and the confidentiality of our trade secrets; potential lawsuits for, or claims of, infringement of third-party intellectual property or challenges to the ownership of our intellectual property; our patents being found invalid or unenforceable; risks associated with international operations; our ability to retain key personnel and to manage our growth; the potential volatility of our common stock; our management and principal stockholders have the ability to control or significantly influence our business; costs and resources of operating as a public company; unfavorable or no analyst research or reports; and securities class action litigation against us.
These and other important factors discussed under the caption "Risk Factors" in our Quarterly Report on Form 10-Q for the three months ended September 30, 2021, and our other reports filed with the SEC, could cause actual results to differ materially from those indicated by the forward-looking statements made in this press release. Any such forward-looking statements represent management's estimates as of the date of this press release. While we may elect to update such forward-looking statements at some point in the future, except as required by law, we disclaim any obligation to do so, even if subsequent events cause our views to change. These forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date of this press release.
Contacts
Investors:
Kendra Sweeney, 239-877-7474
ksweeney@evelobio.com
Media:
Jessica Cotrone, 978-760-5622
jcotrone@evelobio.com