Cook Medical’s Flourish™ is Now Available
BLOOMINGTON, Ind.--(BUSINESS WIRE)-- Cook Medical announced today the availability of the Flourish™ Pediatric Esophageal Atresia device in the U.S. The device was granted authorization by the U.S. Food and Drug Administration (FDA) under the Humanitarian Device Exemption (HDE) for the treatment of pediatric esophageal atresia.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20181114005599/en/

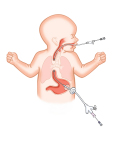
Flourish Pediatric Esophageal Atresia device (Graphic: Business Wire)
Esophageal atresia is a birth defect of the esophagus – the tubular structure that connects the mouth to the stomach. In babies born with this condition, the upper part of the esophagus does not connect to the lower part of the esophagus and the stomach, making it impossible for them to eat normally.
The Flourish Pediatric Esophageal Atresia device uses rare earth magnets that are inserted into the upper and lower ends of the infant’s esophagus. Over the course of several days, the magnets gradually stretch both ends of the esophagus, after which the tissue connects to form an intact esophagus. Previously, this condition could only be repaired surgically, but now doctors have another option.
In order to treat pediatric patients affected by this rare disorder, Flourish received approval from the FDA in 2017. Esophageal atresia affects around 1 in 2,500 newborns.1 Humanitarian Use Device (HUD) designations are reserved for the treatment or diagnosis of a disease or condition that affects fewer than 8,000 individuals in the United States per year. Between the time that FDA granted HUD designation in 2010 and approved the Humanitarian Device Exemption (HDE) for Flourish in 2017, 16 babies were successfully treated.
“We’re thrilled to be able to officially offer this minimally invasive approach for infants suffering with this condition to physicians in the U.S.,” said Barry Slowey, president of Cook Winston-Salem and vice president of Cook Medical’s Endoscopy specialty. “It has been especially rewarding to see the positive effects that Flourish has had on these children. An important part of our culture is to support patient populations that don’t have access to treatment options, which is why we worked with the FDA to establish the HDE pathway.”
For more information about other endoscopy products, visit cookmedical.com/endoscopy.
About Cook Medical
Since 1963 Cook Medical has worked closely with physicians to develop technologies that eliminate the need for open surgery. Today we are combining medical devices, biologic materials and cellular therapies to help the world’s healthcare systems deliver better outcomes more efficiently. We have always remained family owned so that we have the freedom to focus on what we care about: patients, our employees and our communities. Find out more at www.cookmedical.com, and for the latest news, follow us on Twitter, Facebook and LinkedIn.
1. Mahoney L, Rosen R. Feeding difficulties in children with esophageal atresia. Paediatr Respir Rev. 2016;19:21-27.
Device information: Humanitarian Device. Authorized by Federal law for use in the treatment of lengthening atretic esophageal ends and creating an anastomosis with a non-surgical procedure in pediatric patients, up to one year of age with esophageal atresia without a tracheoesophageal fistula (TEF), or in pediatric patients up to one year of age for whom a concurrent TEF has been closed as a result of a prior procedure. This device is indicated for atretic segments < 4 cm apart. The effectiveness of this device for this use has not been demonstrated.
Data supporting the safety and probable benefit of the Flourish device include results from 16 patients who had the Flourish device placed. In the limited data provided, all of the infants had a successful joining of their esophagus, with no remaining gap, within 3 to 10 days after receiving the device. However, 13 of the 16 patients developed a complication that caused a narrowing in their esophagus (anastomotic stricture) that required a balloon dilation procedure, a stent or both to repair. Anastomotic strictures also occur from traditional surgery to repair the condition.
The Flourish device should not be used in patients older than one year or who have teeth, which may damage the oral catheter. The device is also contraindicated in infants who have an existing tracheoesophageal fistula or who have esophageal segments that are more than 4 centimeters apart. This device should not be used for the creation of an anastomosis other than in the esophagus, patients without an established and appropriately sized gastrostomy tract, or patients having gastrostomy site signs of significant infection. Potential complications that may occur when the device is in place include ulceration or tissue irritation around the catheter implanted in the stomach and gum irritation due to pressure from the oral catheter.
Potential late complications following successful anastomosis include gastroesophageal reflux, tracheomalacia, esophageal dysmotility, recurrent asthma and pulmonary infections.
View source version on businesswire.com: https://www.businesswire.com/news/home/20181114005599/en/
Contacts
Marsha Lovejoy
Global Manager, External Corporate Communications, Cook Medical
812.320.6903 (mobile)
812.339.2235, ext. 10-2750 (office)
marsha.lovejoy@cookmedical.com
Source: Cook Medical