Biohaven's BHV-1200, A Multimodal Antibody Therapy Enhancer (MATE), Demonstrates Effective Neutralization Of Multiple Strains Of COVID-19
|
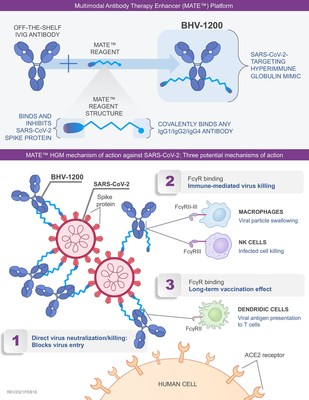
NEW HAVEN, Conn., Feb. 22, 2021 /PRNewswire/ -- Biohaven Pharma Holding Company Ltd. (NYSE: BHVN), a commercial-stage biopharmaceutical company with a portfolio of innovative, late-stage product candidates, today announced that a hyperimmune globulin mimic (HGM) developed with Biohaven's proprietary MATE platform has demonstrated functional binding and neutralization of the SARS-CoV-2 virus, including the strains known as the "English" and "South African" variants (also known as B.1.1.7 and B.1.351, respectively). The preliminary experiments, conducted by Biohaven Labs and an academic collaborator demonstrated that BHV-1200 substantially reduced viral entry into cells. Accelerated development of the COVID-19 MATE program has been supported by the Bill and Melinda Gates Foundation. Vlad Coric M.D., Chief Executive Officer of Biohaven commented, "The battle against COVID-19 –like the virus itself–is dynamic and ever changing. Prophylactic and therapeutic agents with broad specificity against current and future strains will be essential in this ongoing fight to beat the virus. Results from our recent preclinical testing are an exciting advancement for this platform technology, and shows that our lead novel MATE conjugate, BHV-1200, exhibits broad and potent antiviral activity against not only wild type SARS-CoV-2 spike protein but also against mutations of growing clinical relevance, such as those associated with reduced susceptibility to therapeutic monoclonal antibodies and recent SARS-CoV-2 strains." Dr. Coric added, "We believe that BHV-1200 could lead to enhanced efficacy and other benefits over convalescent plasma and alternative antibody-based approaches. Biohaven is excited to advance BHV-1200 into a full clinical development program. We are deeply grateful to the vision and critical funding support provided by The Bill & Melinda Gates Foundation that has accelerated this novel technology towards the clinic." Biohaven's proprietary MATE conjugation technology uses a new class of synthetic peptide binders to target the spike protein of SARS-CoV-2 that are then selectively conjugated to commercially available intravenous immunoglobulin (see Figure 1). The Biohaven synthetic binders for SARS-CoV-2 were designed to establish a much wider area and number of contacts with the spike protein than other agents like monoclonal antibodies. Importantly, the binding and potent neutralizing activity observed with BHV-1200 was consistent across multiple strains of the SARS-CoV-2 virus, including the "English" and "South African" variants. These variants contain multiple mutations in the spike protein that have been reported to reduce the binding and neutralizing activity of currently available antibody-based COVID-19 therapies and sera from SARS-CoV-2 vaccine recipients. In addition, the in vitro data indicate that BHV-1200 may activate important immune system components including antibody-dependent cellular phagocytosis (ADCP) and antibody dependent cellular cytotoxicity (ADCC). Biohaven's proprietary MATE-conjugation technology could also be used against other infectious diseases by changing the targeting moiety of its antibody binders. Dr. Charles Conway, Chief Scientific Officer at Biohaven stated, "Our recent in vitro data provide good evidence that BHV-1200 can neutralize the new strains of SARS-CoV-2 that have recently appeared across the globe. Our MATE-conjugation technology could enable commonly used and commercially available IVIG plasma products, which is not specific for the SARS-CoV-2 virus, to be redirected to target the viral spike protein. We look forward to continue to evaluate the therapeutic utility of this treatment both in the lab and in the clinic." About Biohaven Biohaven is a commercial-stage biopharmaceutical company with a portfolio of innovative, best-in-class therapies to improve the lives of patients with debilitating neurological and neuropsychiatric diseases, including rare disorders. Biohaven's neuroinnovation portfolio includes FDA-approved NURTEC® ODT (rimegepant) for the acute treatment of migraine and a broad pipeline of late-stage product candidates across three distinct mechanistic platforms: CGRP receptor antagonism for the acute and preventive treatment of migraine; glutamate modulation for obsessive-compulsive disorder, Alzheimer's disease, and spinocerebellar ataxia; and myeloperoxidase (MPO) inhibition for multiple system atrophy and amyotrophic lateral sclerosis. Biohaven Labs is a research and discovery arm of the company developing next-generation, bispecific compounds. More information about Biohaven is available at www.biohavenpharma.com. Forward-Looking Statements NURTEC and NURTEC ODT are registered trademarks of Biohaven Pharmaceutical Ireland DAC. MATE is a trademark of Kleo Pharmaceuticals, Inc. (a subsidiary of Biohaven and D/B/A Biohaven Labs) Biohaven Contact
SOURCE Biohaven Pharmaceutical Holding Company Ltd. |
||
Company Codes: NYSE:BHVN |