Adverum Files Definitive Proxy Materials and Mails Letter to Stockholders Detailing Strength of Team and Acceleration Toward Commercialization of ADVM-022
- Urges Stockholders to Vote the WHITEProxy Card “FOR ALL” of Adverum’s Three Highly Qualified, Diverse and Independent Directors: Dawn Svoronos, Reed Tuckson, M.D. and Tom Woiwode, Ph.D.
- Annual Meeting to Be Held on May 12, 2021
REDWOOD CITY, Calif., April 15, 2021 (GLOBE NEWSWIRE) -- Adverum Biotechnologies, Inc. (Nasdaq: ADVM), a clinical-stage gene therapy company targeting unmet medical needs in ocular and rare diseases, today announced that it has filed definitive proxy materials with the U.S. Securities and Exchange Commission in connection with Adverum’s 2021 Annual Meeting of Stockholders (“Annual Meeting”), to be held virtually on May 12, 2021 at 8:30 a.m. Pacific Time. Adverum stockholders of record at the close of business on April 14, 2021 are entitled to vote at the Annual Meeting.
The Adverum Board of Directors unanimously recommends that stockholders vote the WHITE proxy card FOR Adverum’s three highly qualified directors standing for election - Dawn Svoronos, Reed Tuckson, M.D. and Tom Woiwode, Ph.D.
In conjunction with the definitive proxy filing, Adverum is mailing a letter to stockholders detailing the significant clinical advancements the Board and management team are executing to accelerate toward commercialization and create value for stockholders. The full text follows and can be found on the investor section of the Company's website at https://investors.adverum.com/shareholder-services/annual-meeting.
April 15, 2021
VOTE THE WHITE PROXY CARD TODAY “FOR ALL” OF ADVERUM’S THREE HIGHLY QUALIFIED, DIVERSE AND INDEPENDENT DIRECTORS
REJECT SONIC’S SELF-SERVING ATTEMPT TO TAKE CONTROL OF THE ADVERUM BOARD
Dear Fellow Stockholder,
You have an important decision to make regarding the future of your investment in Adverum. At our Annual Meeting of Stockholders to be held on May 12, 2021, you will be asked to elect the directors who you believe are most qualified to oversee the execution of Adverum’s long-term strategy as we accelerate the development and commercial launch plans for ADVM-022, our one-time, in-office advanced gene therapy product for the treatment of the leading causes of blindness globally.
Adverum is making tremendous progress implementing plans across the business to support a successful development strategy and commercial launch. We urge you to ensure this momentum continues by supporting Adverum’s three highly qualified directors standing for election at this year’s Annual Meeting and vote your shares FOR ALL of Adverum’s director nominees - Dawn Svoronos, Reed Tuckson, M.D. and Tom Woiwode, Ph.D. - on the WHITE proxy card today.
Your vote is especially important this year given that The Sonic Fund II, L.P. (“Sonic”) – an activist stockholder – has filed proxy materials to run a competing slate of candidates for election to the Adverum Board.
Over the last several years, we have maintained an open and constructive dialogue with Sonic, including working collaboratively with Sonic in May of 2019 to appoint to our Board two new directors proposed by Sonic. The Board and management team of Adverum spent significant time, both before and after the settlement agreement in 2019, engaging with Sonic’s principal, Lawrence Kam. In our conversations, we have reiterated our openness to evaluating suggestions from all stockholders to drive long-term value creation.
Despite Adverum’s good faith efforts to take feedback and incorporate Mr. Kam’s perspectives as reasonably as possible for over the past two years, it has now become clear that Mr. Kam is committed to trying to take more control than is in the best interest of all Adverum stockholders.
Adverum’s significant clinical advancements with ADVM-022 have been guided by a strong Board and leadership team that has been meaningfully refreshed over the last two years. Given the positive action and progress underway, Sonic’s proxy contest is unnecessary, distracting, costly and not in the best interest of all stockholders. Sonic’s proposed nominees, if elected, together with its two designees appointed in 2019, would constitute more than half of the Board. We do not believe allowing an approximately 7% stockholder to hand-pick a majority of the Board is in the best interest of our stockholders, our patients or any of our other stakeholders.
While Sonic has openly criticized Adverum’s leadership and strategy, Sonic has not articulated a single strategic plan or provided details on what it intends to do with your company if Sonic gains control of the Board. All Mr. Kam has done is make personal attacks on members of our Board and leadership team with no substantiation or basis in fact and without providing constructive input.
Your vote on the WHITE proxy card in advance of our Annual Meeting of Stockholders is critically important, no matter how many shares you own. Not doing so would put at risk our accelerating momentum and execution of a clear strategic plan to establish our leading ocular gene therapy program as a one-time treatment that preserves patient sight for life.
Transformative Gene Therapy
Adverum has an incredible opportunity ahead of it, and one that we work tirelessly every day to bring to fruition for the benefit of patients. Our market opportunity is large and rapidly growing:
- Approximately nine million patients live with wet age-related macular degeneration (AMD) or diabetic macular edema (DME) treatable with anti-VEGF worldwide1; and
- $11.5 billion was spent globally on approved anti-VEGF therapies in 2020,2 with 12% of the U.S. Medicare Part D budget spent on anti-VEGF therapies annually3.
The current standard of care for wet AMD and DME requires an eye injection every four to eight weeks, often requiring a full day for treatment and recovery, in order to avoid vision loss. Therefore, it should come as no surprise that Adverum’s extensive market research and frequent discussion with retina specialists and patients reveal that the largest unmet need for patients with wet AMD is for a durable treatment that reduces the frequency of injections and provides increased efficacy, improved ease of administration and better control of macula fluid.
ADVM-022, our program designed to treat wet AMD and DME with a single in-office intravitreal injection, is the only investigational non-surgical treatment option that has demonstrated long-term durability up to two years. We believe that ADVM-022 is uniquely positioned, unlike any other treatment on the market or in development, as an advanced gene therapy product with the potential to be a one-and-done approach for millions of people living with wet AMD and DME globally.
Based on the results in our OPTIC trial to date and our constructive discussion with the U.S. Food and Drug Administration (FDA), we are planning to accelerate our clinical development program for ADVM-022 by conducting two global Phase 3 trials in wet AMD targeted to initiate in parallel in the fourth quarter of 2021. This supports shortened timelines and a clear path for Biologics License Application submission in 2024. We continue to work with the top retinal specialists in the field as we conduct clinical trials for ADVM-022 in wet AMD and DME.
To date, ADVM-022 in wet AMD has demonstrated favorable efficacy and safety profiles. In the OPTIC trial, patients who received 2 x 10^11 vg/eye of ADVM-022 experienced an 85% reduction in annualized anti-VEGF injections and two-thirds remained supplemental anti-VEGF injection free with median follow up of 48 weeks4.
The safety of patients is our top priority. While data shows that ADVM-022 continues to be well tolerated with ocular cell grades and steroid eye drop use decreasing over time, we have convened multiple advisory boards of experts to optimize the prophylaxis regimen and are following our advisors’ recommendations. We are committed to pursuing multiple paths to improve results with respect to inflammation given the treatment durability observed beyond two years in patients who received a single injection of ADVM-022.
In addition, Adverum announced in January 2021 the completion of patient enrollment in the INFINITY Phase 2 trial of ADVM-022 for DME, which we achieved in under six months despite the challenges posed by the COVID-19 pandemic. Adverum expects to present clinical data from this trial in the second half of 2021.
Accelerating Toward Commercialization
Your Board and management team are taking decisive action to accelerate our ADVM-022 development and commercialization plans to launch our first mass-market gene therapy to treat millions of patients with wet AMD and DME if ADVM-022 receives FDA approval. We are focused on execution, with the goal of delivering this transformative gene therapy to patients globally as early as possible.
In January 2021, we announced a significant investment in a new state-of-the-art Good Manufacturing Practices (GMP) commercial facility in Durham, North Carolina, which spans 174,00 square feet and is expected to be production-ready by the end of 2023. This facility capitalizes on our internal AAV-gene therapy manufacturing expertise while providing security and flexibility as we move forward to supply large markets around the world.
In addition, Adverum has all process development, assay development and quality control in-house and is partnering with contract manufacturing organizations to manufacture clinical and additional commercial supply. We use a highly scalable baculo/Sf9 suspension process, having scaled up from 50 liters to 200 liters, and are now in the process of scaling up to 1,000 liters.
Importantly, given the complexity of gene therapy manufacturing, controlling the entirety of the manufacturing process has proven to be critical in maximizing the value of the therapy long-term. Owning an in-house GMP facility that can provide dedicated commercial supply at scale is a core differentiator for gene therapy companies, as it meaningfully de-risks the ability to get these critical therapies to patients. Gene therapy companies with owned manufacturing facilities are further rewarded for this de-risking through higher market valuations and greater strategic interest over the long-term.
Adverum maintains a disciplined approach to capital allocation and will continue to invest in advancing ADVM-022 toward commercialization and further building out our manufacturing capabilities – with the goal of creating long-term value for our stockholders.
Delivering Value for Our Stockholders
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/2496e46f-9eed-46c0-8dc8-5a24a3114549
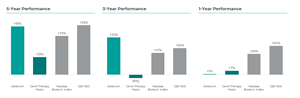
Even before commercialization, Adverum’s efforts have already delivered value for our stockholders. Adverum has delivered five- and three-year total stockholder return of 91% and 70% respectively, significantly outperforming gene therapy peers.5
As Adverum continues to report positive interim data and continues to achieve pre-commercialization, clinical and development milestones throughout the rest of 2021 and beyond, we expect to drive significant value for our stockholders.
Importantly, additional clinical data will be presented for Cohorts 1-4 in the OPTIC clinical trial of ADVM-022 intravitreal injection gene therapy in wet AMD during the Association for Research in Vision and Ophthalmology 2021 Virtual Meeting in early May.
Your Recently Refreshed Board is Highly Qualified, Engaged and Diverse: Seven New Independent Directors Added in the Last Two Years, Including Two of Sonic’s Designees
Adverum has an experienced, well-rounded and independent Board that benefits from the right mix of skills to position it for success as we navigate upcoming clinical and operational milestones. Our world-class directors are industry leaders that are actively engaged in overseeing Adverum’s strategic execution and are holding management accountable.
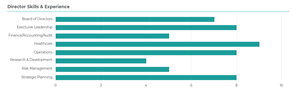
Seven of our nine continuing directors are independent, eight are non-employees and all of our directors bring expertise critical to our business, including gene therapy and other drug development, ophthalmology, global commercialization, R&D, policy and managed care expertise, as well as finance and accounting, governance and risk management.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/d599c5d4-e40d-41c0-a843-7ebe9b27037d
The Adverum Board is also committed to ongoing refreshment to ensure a best-in-class and diverse make up. In the last two years, the Board has appointed seven new non-employee directors, three in May 2019 and four thereafter, including Ms. Svoronos and Dr. Tuckson in December 2020 and February 2021, respectively. We have a rigorous selection process, which has produced a highly diverse Board, with each member bringing a specific skillset that is aligned with our long-term strategic priorities, and ideal for overseeing the critical process of preparing for commercialization.
While Sonic’s nominees are not right for Adverum, the Board is committed to continuing its refreshment efforts and has made it a priority to recruit a high-quality director with product and commercial gene therapy experience to be named in 2021. Notably, we have engaged with our stockholders about top candidates and already interviewed candidates to fill this slot, and strongly believe that this is a superior process to relying solely on input from one stockholder.
Your Board’s nominees for the 2021 Annual Meeting – Ms. Svoronos, Dr. Tuckson and Dr. Woiwode – collectively bring decades of public company board experience and critical sector expertise.
- Ms. Svoronos is a commercialization expert and has three decades of global biopharmaceutical industry experience, spanning the United States, Canada, Europe, and Asia. During her over two-decade career at Merck, Ms. Svoronos led and/or was involved in dozens of pharmaceutical product launches in key markets around the world, many of which were mass market opportunities. Ms. Svoronos serves on the Board of four companies, including Global Blood Therapeutics, Inc., and PTC Therapeutics.
- Dr. Tuckson is an esteemed healthcare executive and brings extensive policy knowledge from his experience in multiple facets of the healthcare industry, including a 13-year tenure in senior leadership positions at UnitedHealth Group, where he served as chief of medical affairs. He has a strong understanding of the complex reimbursement environment and proven track record of enhancing patient access, as exemplified by his recent COVID-19 vaccine efforts in disproportionally impacted minority populations.
- Dr. Woiwode, an Adverum director since 2016, has been with Versant Ventures since 2002, serving as a venture partner since 2008 and a managing director since 2014. Versant Ventures is a significant stockholder of Adverum. Dr. Woiwode has served in a number of operating roles, most recently as the chief operating officer of Okairos, which was sold to GlaxoSmithKline plc. Dr. Woiwode serves as a director at Aligos Therapeutics, Inc., Gritstone Oncology, Inc., Passage BIO, Inc. and several private life sciences companies.
Our commitment to value creation, diversity and ESG principles is embedded in our core and helps drive long-term value creation for stockholders. The entire Adverum Board is dedicated to ensuring the management team and long-term strategy is mission driven. Public health is the essence of who we are, and the Board considers this one of our most important ESG values.
Further, our ability to help people extends well beyond working to treat vision loss and eye disorders. Our people are our brain trust. We believe our culture is a key advantage and we are committed to supporting our employees. We believe brands are built from the inside out, and our employees are the cornerstone of our business.
Refreshed, World-Class Management Team in Place to Navigate Upcoming Milestones and Successfully Drive Toward Commercialization
We have a refreshed management team in place to drive our strategy, including Chief Executive Officer Laurent Fischer, M.D., President and Chief Financial Officer Leone Patterson, Chief Technology Officer Angela Thedinga, MBA, MPH, and Chief Business Officer Christopher DeRespino.
In addition, a new Chief Scientific Officer has accepted Adverum’s offer and will be announced in the coming weeks, and we are conducting a comprehensive search process to identify our next Chief Medical Officer.
Dr. Fischer and Ms. Patterson have been instrumental in building a standout team filled with the right mix of business, entrepreneurial and specialized technical expertise – all of which is critical to building a world-class gene therapy organization ready for commercial launch. We are confident that your entire leadership team is uniquely positioned to execute Adverum’s strategy and drive long-term value for our stockholders.
Adverum Has a Long History of Good Faith Engagement with Sonic
Adverum has carried on an active dialogue with Mr. Kam since 2019 when he was first introduced to our current Chairman, Patrick Machado, by Dr. Thomas Chalberg. Dr. Chalberg was the founder and original chief executive officer of Avalanche Biotechnologies, Inc., one of the two companies that merged in 2016 to form Adverum. Although Adverum has an entirely new Board, management team and development program, it has not completely shaken off the legacy of pre-merger Avalanche’s poor performance. While Dr. Chalberg has had no role with Adverum for many years – other than as a stockholder – he and Mr. Kam have relentlessly engaged with the Adverum Board on matters related to Board director appointments, the hiring of senior management, long-term strategy and capital allocation.
During this long engagement, Mr. Kam has made numerous suggestions that reflect questionable judgment, including the following:
- Mr. Kam expressed his desire for Mr. Chalberg to be the CEO of Adverum, stating to one of the directors he placed on our Board in 2019 that “He [Dr. Chalberg] really should be Executive Chairman, CEO, or whatever but has other commitments and knows that his association with Avalanche 1.0 does not help shake the Avalanche 2.0 image.’’ Two of Mr. Kam’s nominees also have ties to Avalanche, one as an employee and one as a scientific advisory board member.
- Mr. Kam repeatedly proposed that Adverum divert resources to pursue a $20 million lead investment in the initial financing round of a start-up gene therapy company using a different technology in a different therapeutic area from what Adverum is pursuing. Notably, to our knowledge, this company has not been financed or progressed meaningfully.
Despite Mr. Kam’s questionable actions, your Board and management team have remained committed to open engagement and to considering Mr. Kam’s ideas. In fact, on April 6th, our Nominating and Corporate Governance Committee offered to interview Sonic’s proposed candidates. Unfortunately, after more than a week of silence, Sonic’s legal counsel responded to Adverum’s request with questions rather than an answer.
Sonic’s Attempt to Replace Three Highly Qualified and Diverse Independent Directors with Its Own Nominees Puts at Risk Operational Execution and Is Not in the Best Interests of Stockholders
Not only would Sonic’s nominees, if elected, together with its two nominees appointed in 2019, constitute more than half of the Board, their election would significantly diminish the diversity of expertise and experience on the Board that has been so critical to our recent efforts towards commercial readiness. We believe that allowing Sonic to hand-pick a majority of the Board would significantly disrupt our progress toward achieving our upcoming milestones and delay the value opportunity of ADVM-022.
Sonic is putting at risk Adverum’s laser focus on operational execution as well as our ability to retain and recruit world-class talent. While Sonic is focused seemingly on reassembling a team from the past, Adverum is staying focused on the future and accelerating toward commercialization. Stockholders should not allow Sonic to impede our momentum.
Your Vote Is Extremely Important! Vote the WHITE Proxy Card Today to Protect Your Investment
Vote the Enclosed WHITE Proxy Card Today “FOR ALL” Three of Adverum’s Highly Qualified and Diverse Director Nominees
Adverum is well-positioned to capitalize on the opportunities ahead. Your Board and management team are focused on accelerating the development and future commercial launch plans for ADVM-022, and we firmly believe we have the right directors in place to do just that.
We urge you to use the enclosed WHITE proxy card to vote today "FOR" ALL three of Adverum's nominees listed on the WHITE proxy card: Ms. Svoronos, Dr. Tuckson and Dr. Woiwode. Simply follow the easy instructions on the enclosed proxy card to vote by telephone, by Internet or by signing, dating and returning the WHITE proxy card in the postage-paid envelope provided. Please disregard any green proxy card you get from Sonic.
We appreciate your continued support as we work to drive value for ALL stockholders.
Sincerely,
The Adverum Board of Directors
Advisors
Cooley LLP and Skadden, Arps, Slate, Meagher & Flom LLP are serving as legal advisors, and Centerview Partners LLC is serving as financial advisor to Adverum.
About Adverum Biotechnologies
Adverum Biotechnologies (Nasdaq: ADVM) is a clinical-stage gene therapy company targeting unmet medical needs in serious ocular and rare diseases. Adverum is advancing the clinical development of its novel gene therapy candidate, ADVM-022, as a one-time, intravitreal injection for the treatment of patients with wet age-related macular degeneration and diabetic macular edema. For more information, please visit www.adverum.com.
Forward-looking Statements
Statements contained in this press release regarding the events or results that may occur in the future are “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. Such statements include, but are not limited to, statements regarding Adverum’s expectations that its two global Phase 3 trials in wet AMD will initiate in parallel in the fourth quarter of 2021, that it will submit a Biologics License Application in 2024, that it expects to present clinical data from the INFINITY trial in the second half of 2021, that its commercial facility in Durham, North Carolina is expected to be production-ready by the end of 2023, that it will present additional data from the OPTIC clinical trial in early May 2021, and that it will announce its new Chief Scientific Officer in the coming weeks and may add an additional director in 2021. Actual results could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties, which include risks inherent to, without limitation: Adverum’s novel technology, which makes it difficult to predict the time and cost of product candidate development and obtaining regulatory approval; the results of early clinical trials not always being predictive of future results; and the potential for future complications or side effects in connection with use of ADVM-022. Risks and uncertainties facing Adverum are described more fully in Adverum’s Annual Report on Form 10-K for the year ended December 31, 2020 and any subsequent filings with the SEC under the heading “Risk Factors.” All forward-looking statements contained in this letter speak only as of the date on which they were made. Adverum undertakes no obligation to update such statements to reflect events that occur or circumstances that exist after the date on which they were made.
Important Information
Adverum Biotechnologies, Inc. (“Adverum”) has filed a definitive proxy statement and form of associated WHITEproxy card with the U.S. Securities and Exchange Commission (the “SEC”) in connection with the solicitation of proxies for Adverum’s 2021 Annual Meeting (the “Proxy Statement”). Adverum, its directors and certain of its executive officers and employees will be participants in the solicitation of proxies from stockholders in respect of the 2021 Annual Meeting. Information regarding the names of Adverum’s directors, executive officers and employees and their respective interests in Adverum by security holdings or otherwise is set forth in the Proxy Statement. Details concerning the nominees of Adverum’s Board of Directors for election at the 2021 Annual Meeting are included in the Proxy Statement. BEFORE MAKING ANY VOTING DECISION, INVESTORS AND STOCKHOLDERS OF ADVERUM ARE URGED TO READ ALL RELEVANT DOCUMENTS FILED WITH OR FURNISHED TO THE SEC, INCLUDING THE ADVERUM’S DEFINITIVE PROXY STATEMENT AND ANY SUPPLEMENTS THERETO AND ACCOMPANYING WHITEPROXY CARD, BECAUSE THEY CONTAIN IMPORTANT INFORMATION. Investors and stockholders can obtain a copy of the Proxy Statement and other relevant documents filed by Adverum free of charge from the SEC’s website, www.sec.gov. Stockholders may also contact Innisfree M&A Incorporated with questions or requests for additional copies of the proxy materials by calling toll free at (877) 750-9496.
1 MarketScope Retina Report for 2020 in US, EU, JP
2 Based on 2020 public filings from Regeneron, Roche and Novartis
3 Medicare Spending on Anti-Vascular Endothelial Growth Factor Medications, August 2018
4 Adverum press release on March 1, 2021
5 FactSet as of March 31, 2021; Gene Therapy peers reflect median of Abeona, AGTC, Amicus, AVROBIO, Gensight, Homology, Krystal, MeiraGTx, Orchard, Passage Bio, REGENXBIO, Rocket, Sangamo, Solid Biosciences, uniQure and Voyager.
Investor Relations Contact Myesha Lacy Vice President, Investor Relations and Corporate Communications Adverum Biotechnologies, Inc. T: 650-649-1257 E: mlacy@adverum.com Or Innisfree M&A Incorporated Scott Winter / Gabrielle Wolf T: 212-750-5833 Media Contact Andrea Cohen Sam Brown Inc. T: 917-209-7163 E: andreacohen@sambrown.com Or Joele Frank, Wilkinson Brimmer Katcher Dan Moore T: 212-355-4449