Torax Medical Release: New Study Validates LINX As A Preferred New Treatment For Gastroesophageal Reflux Disease
ST. PAUL, Minn., Dec. 1, 2015 /PRNewswire/ -- Torax Medical, Inc., a leader in therapeutic interventions for sphincter-related diseases, announced today the release of new data from a 415 patient, multi-institutional study, comparing magnetic sphincter augmentation to laparoscopic Nissen fundoplication (LNF) for control of reflux. The study was led by Brian Louie, MD, Director, Thoracic Research and Education and colleagues from the Swedish Medical Center in Seattle, Washington. Collaborating centers were led by John Lipham, MD, Chief of Upper GI and General Surgery at Keck School of Medicine of USC in Los Angeles, California and Paul Taiganides, MD, Director of the Heartburn Treatment Center at Knox Community Hospital in Mount Vernon, Ohio.
Experience the interactive Multimedia News Release here: http://www.multivu.com/players/English/7698451-torax-linx-new-study-gerd/
The authors compared patients with gastroesophageal reflux disease (GERD) undergoing anti-reflux surgery with the LINX® Reflux Management System (LINX) to the standard of care, LNF at one year. The LINX device is based on using special properties of magnetic attraction to restore the barrier function of the esophageal sphincter by supporting and maintaining the natural anatomy with an expandable ring of magnetic beads. The current gold standard of care, LNF requires the patient's stomach anatomy to be permanently altered to create a new valve using stomach tissue. This approach is commonly associated with the patient losing the ability to vomit and belch. The results demonstrated that LINX achieves similar improvements in symptomatic relief of reflux with fewer side effects. Additionally, patients who underwent the LINX procedure were more likely to report they would undergo the procedure again.
"This study puts LINX as a first-line therapy for GERD as it preserves the patient's gastric anatomy and minimizes side effects which are well established limitations of the current standard of care Nissen fundoplication" said Dr. Brian Louie. "LINX is a needed and effective surgical treatment option for patients with GERD that are not adequately controlled with medical therapy." The study results were published in Surgical Endoscopy (http://www.ncbi.nlm.nih.gov/pubmed/26541740).
The Disease
Gastroesophageal Reflux Disease (GERD) is a chronic, often progressive disease resulting from a weak lower esophageal sphincter that allows harmful gastric fluid to reflux into the esophagus, resulting in both pain and injury to the esophageal lining. GERD is associated with a pre-cancerous condition known as Barrett's esophagus, which increases the risk of esophageal cancer. Symptoms of GERD include heartburn and regurgitation, often associated with chronic sleep disruption, and may also include persistent cough, excessive throat clearing, hoarseness and a feeling of a "lump" in the throat. Acid reflux medications, such as Prevacid®, Nexium®, and Prilosec®, affect gastric acid production, but do not repair the sphincter defect, allowing continued reflux. Anti-reflux surgery called Nissen fundoplication reconstructs a new reflux barrier using a portion of the patient's stomach which is wrapped around the lower portion of the esophagus.
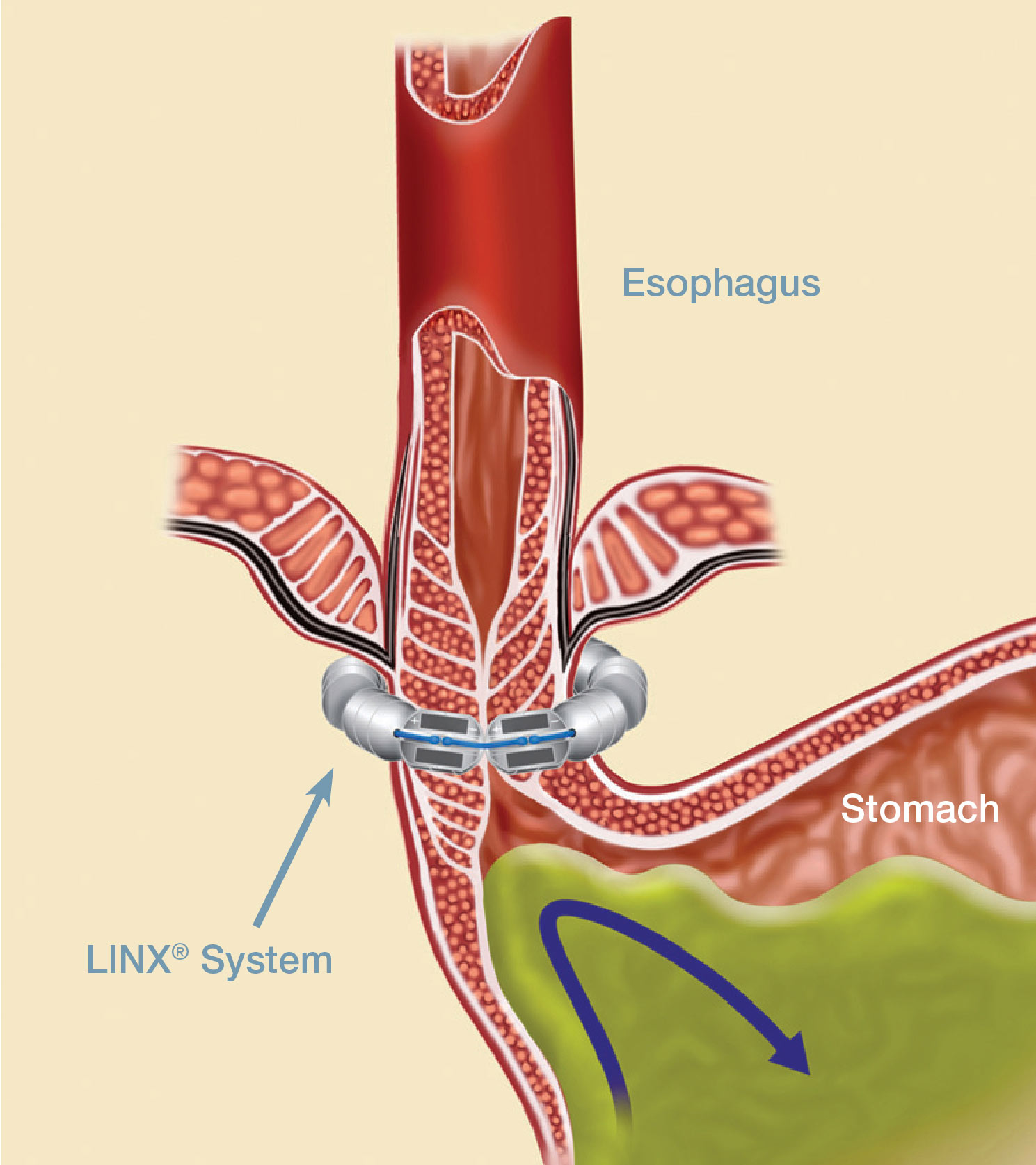
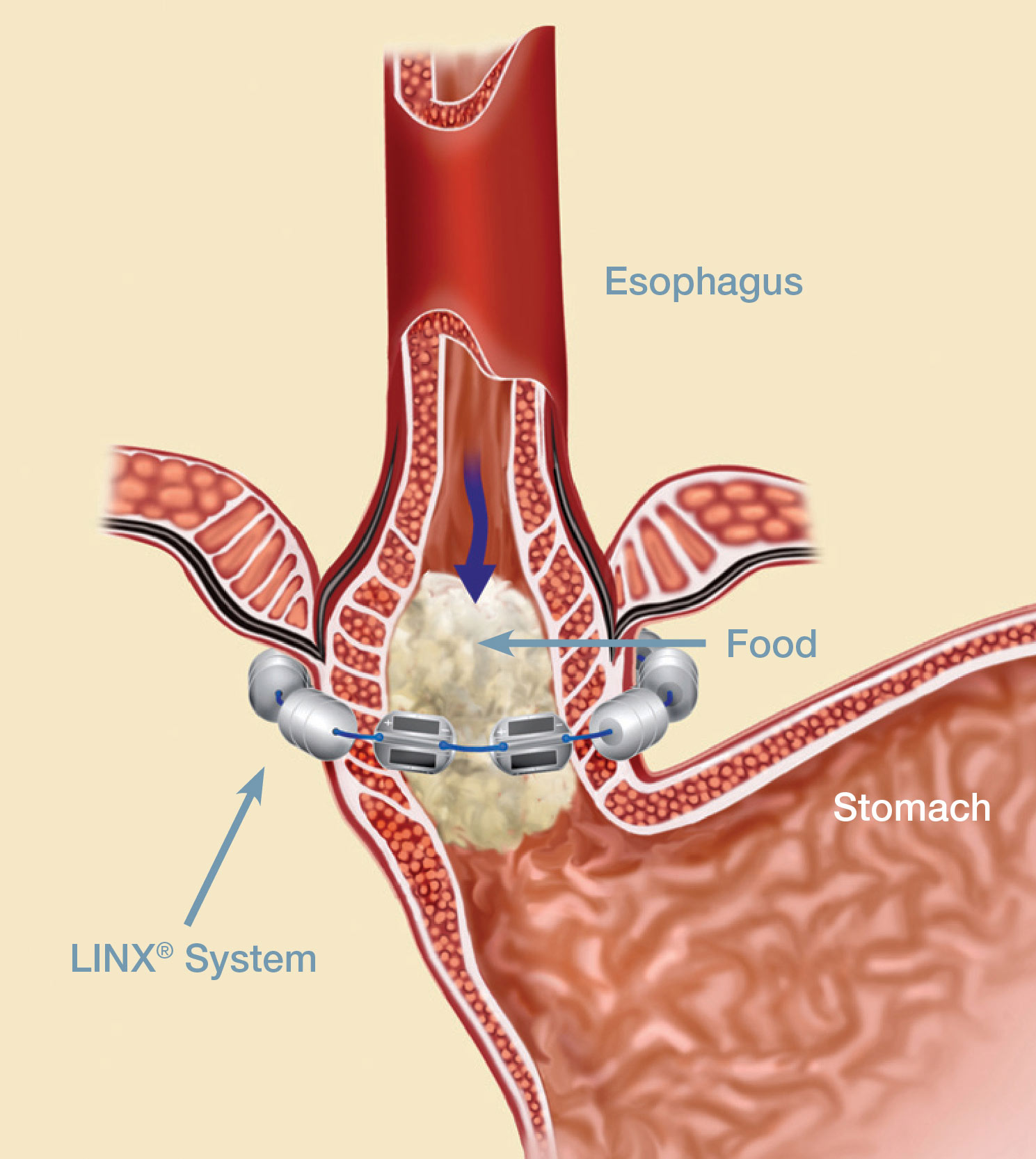
The LINX Reflux Management System
LINX is a small implant comprised of interlinked titanium beads with magnetic cores. The magnetic attraction between the beads augments the existing esophageal sphincter's barrier function to prevent reflux. The device is implanted using a standard minimally invasive laparoscopic procedure and is an alternative to the more anatomically disruptive fundoplication, commonly used in surgical anti-reflux procedures. The LINX Reflux Management System is indicated for those patients diagnosed with GERD as defined by abnormal pH testing, and who continue to have chronic GERD symptoms despite maximum medical therapy for the treatment of reflux.
LINX does require a surgical procedure and is associated with potential risks, contraindications and life style modifications. For more information on LINX, including a statement of risks, please visit www.linxforlife.com.
About Torax Medical
Torax Medical, Inc. is a privately-held medical device company headquartered in St. Paul, Minnesota that develops and markets products designed to treat sphincter disorders utilizing its technology platform, Magnetic Sphincter Augmentation (MSA). Torax Medical is currently marketing the LINX® Reflux Management System for the treatment of GERD in the U.S. and Europe and the FENIX® Continence Restoration System for the treatment of Fecal Incontinence (FI) in Europe. For more information, please visit www.toraxmedical.com.
Maggie Wallner
Torax Medical, Inc.
info@toraxmedical.com
651-361-8900

To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/new-study-validates-linx-as-a-preferred-new-treatment-for-gastroesophageal-reflux-disease-300185850.html
SOURCE Torax Medical, Inc.

