Samumed Presents At The Annual Meeting Of The American Academy of Dermatology Safety And Efficacy Results From Its Phase 2 Androgenetic Alopecia (AGA) Trial
SAN DIEGO, March 7, 2016 /PRNewswire/ -- Samumed, a leader in tissue regeneration, presented results from its Phase 2 AGA trial during the 74th Annual Meeting of the AAD in Washington, DC, at the "Late-breaking Research ForumsProcedural Dermatology" session. The presentation slides can be found at www.samumed.com.
SM04554 appeared to be safe and well-tolerated. There were no serious adverse events (SAE) observed in the treatment groups, and the incidence of adverse events (AE) was similar between treatment and control groups.
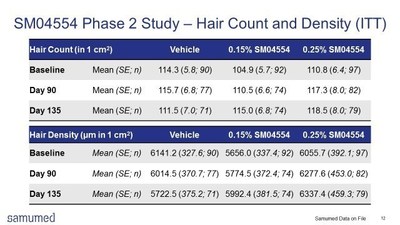
Photo - http://photos.prnewswire.com/prnh/20160307/340958-INFO
As presented in the slide reproduced above, over the 135 day study, both treatment arms showed an increase in hair count and hair density objectively measured by macrophotography. By contrast, the vehicle group showed a decline in both measures. Under the a priori statistical analysis plan, between Day 90 and Day 135, the 0.15% treatment group had a statistically significantly higher increase in both hair count and hair density, as compared to vehicle (Figure 1).
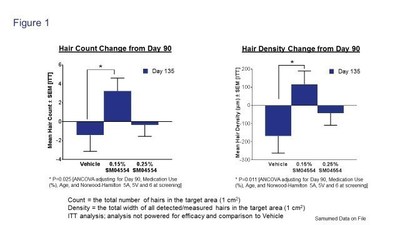
Photo - http://photos.prnewswire.com/prnh/20160307/340959-INFO
Samumed's investigational drug is a topical solution of its novel small molecule compound SM04554. The 302-subject, multi-center, randomized (1:1:1), double-blind, vehicle-controlled Phase 2 trial studied the safety, tolerability and efficacy of two different concentrations (0.15% and 0.25%) of SM04554 in male subjects with AGA between the ages of 18 and 55 with Norwood-Hamilton Classification scales of 4, 5, 5A, 5V, or 6. The study involved a 90-day once-a-day treatment period and a 45-day post-treatment follow up.
ABOUT SAMUMED, LLC
Based in San Diego, CA, Samumed (www.samumed.com) is a pharmaceutical platform company focused on advancing regenerative medicine and oncology applications through research and innovation. Samumed has discovered new targets and biological processes in the Wnt pathway, allowing the team to develop small molecule drugs that potentially address numerous degenerative conditions as well as many forms of cancer.
Media Contact:
Chrissy Randall
crandall@brunswickgroup.com
202-393-7337
To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/samumed-presents-at-the-annual-meeting-of-the-american-academy-of-dermatology-aad-safety-and-efficacy-results-from-its-phase-2-androgenetic-alopecia-aga-trial-300231624.html
SOURCE Samumed, LLC

