NYU Langone Medical Center Release: High Blood Pressure Drugs May Counter Leading Cause Of Death In Malaria Patients
NEW YORK, Sept. 20, 2016 /PRNewswire-USNewswire/ -- Adding a popular high blood pressure drug to standard malaria treatment more than tripled the survival rate of infected mice. That is the finding of a study led by researchers at NYU Langone Medical Center and published Sept. 19 in the Journal of Clinical Investigation.
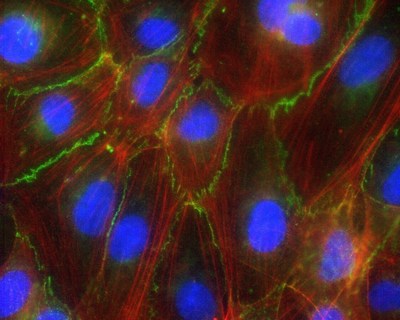
Malaria is a mosquito-borne infection where a bite passes a parasite into the bloodstream. Eliminated from the United States in the 1950s, the disease still kills hundreds of thousands each year, mostly children in Sub-Saharan Africa.
The study results address cerebral malaria, where the parasite causes swelling and bleeding in the brain. Around one percent of the 216 million people infected globally each year develop cerebral malaria. Of those, 15 to 20 percent die, likely representing the majority of the 438,000 deaths attributed to malaria last year, say the study authors.
"About one in five patients with cerebral malaria die within 48 hours of being admitted to the hospital, the time it takes for the parasite-killing drug to take effect," says senior study author Ana Rodriguez, PhD, associate professor in the Department of Microbiology at NYU Langone. "If we could add a drug that stopped hemorrhages during that window, it would buy time and save lives."
In experiments, mice were divided into groups either treated only with chloroquine, a drug commonly used to kill the parasite, or with chloroquine in combination with one of two anti-hypertensive treatments. While just 18 percent of mice treated only with chloroquine survived, 65 percent of mice also given irbersartan, an angiotensin receptor 1 blocker, survived, as did 73 percent of mice also treated with C21, an experimental drug that increases signaling through angiotensin receptor 2.
The team also found that infected mice treated with one of the two angiotensin-influencing drugs experienced fewer, smaller hemorrhages, and in most cases fully recovered.
Deadly Cycle
It was known going into the study that blood cells infected with malaria produce more of a protein that makes them stick to blood vessel walls. Also known was that malaria parasites multiply inside blood cells, which finally burst after about two days to release more parasites.
The new study found that the bursting of infected blood cells, by showering their contents on the vessel walls they are stuck to, sends signals that interfere with the ability of wall-lining cells to cling to each other. Each cell in the wall holds on less tightly to its neighbors, opening gaps through which first blood serum and later whole blood can escape into brain tissue.
The research team used a genetically engineered version of the malaria parasite that enabled them to control the timing of blood cell bursts by adding a trigger molecule. Endothelial cell-cell junctions were fine before the blood cells burst, but compromised afterward.
Some factor from the burst blood cells causes a protein called beta-catenin to break away from the complex that maintains the grip of one endothelial cell on its neighbors, say the authors. Once free from its complex, beta-catenin travels to the nucleus to activate genes that cause a wall-lining cell to further detach from neighboring cells.
Both angiotensin-influencing drugs stopped the activation of beta-catenin and prevented the disruption of blood vessel wall integrity. Angiotensin is a hormone that controls many factors related to blood pressure, sending its signal by fitting into receptor proteins on cells surfaces, like a lock into a key. Study author Julio Gallego-Delgado, PhD, a researcher in the Rodriguez's team, had seen a 2010 genetic study suggesting that biochemical pathways related to this hormone may be protective against cerebral malaria, and decided to test the idea.
Next steps for the team will be to determine the exact molecules coming from burst blood cells that signal to beta catenin in vessel walls, and to conduct a clinical trial that tests the effects of the study drugs in cerebral malaria patients.
Along with Rodriguez and Gallego-Delgado, study authors within the Department of Microbiology at NYU Langone included Upal Basu-Roy, Maureen Ty, Cristina Fernandez-Arias, Alexandru Movila, Pollyanna Gomes, Wenyue Xu, Innocent Edagha, and Samuel Wassmer. Also study authors were Matilde Alique and Marta Ruiz-Ortega of the Cellular Biology in Renal Diseases Laboratory at the Universidad Autónoma Madrid; and Thomas Walther of the Department of Pediatric Surgery & Department Obstetrics, Division of Women and Child Health, Center for Perinatal Medicine, University of Leipzig, Germany.
Funding for the study was provided by National Institutes of Health grants 1R01HL130630, 5T32AI007180-18 30 and U19AI089676-01S; as well as grants from Fundacion Española para la Ciencia y la Tecnologia, Ministry of Education of Spain, Red de Investigación Renal (REDinREN; RD12/0021), and Comunidad de Madrid (Fibroteam; S2010/BMD-2321). Additional support came from the Dana Foundation Program in the Neuroimmunology of Brain Infections and Cancers.
Media contact: Greg Williams, gregory.williams@nyumc.org, 212-404-3533
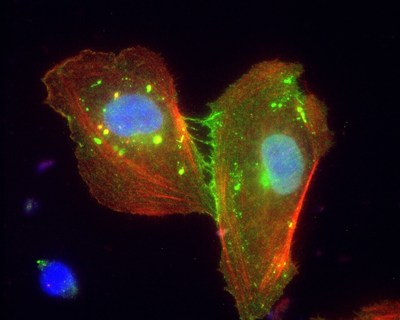

Photo - http://photos.prnewswire.com/prnh/20160920/409940
Photo - http://photos.prnewswire.com/prnh/20160920/409939
Logo - http://photos.prnewswire.com/prnh/20150715/237434LOGO
To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/high-blood-pressure-drugs-may-counter-leading-cause-of-death-in-malaria-patients-300331219.html
SOURCE NYU Langone Medical Center

