IceCure Medical Ltd. Release: National Multi-Center Clinical Trial Of Non-Surgical Breast Cancer Treatment Enrolls First Patients
MEMPHIS, Tenn., Oct. 21, 2014 /PRNewswire/ -- IceCure Medical, Inc. announced today the successful treatment of the first two patients in a multi-centered clinical trial using cryoablation (exposure to extreme cold temperatures) to treat breast cancer without surgery. Dr. Linsey Gold in conjunction with Regional Medical Imaging, Flint, MI and Dr. Richard Fine of The West Clinic, Memphis, TN completed their first cases this week. With these and other leaders in cancer treatment using IceCure's technology, the ICE3 trial is significantly expanding the data on cryoablation and may create a paradigm shift in the treatment of breast cancer.
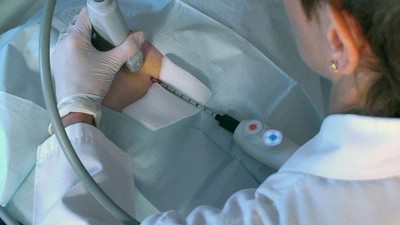
"Breast cancer awareness month during October is the perfect time to kick off this important initiative. The treatment of breast cancer has evolved to include not only mastectomy but also breast-conserving lumpectomy. Now cryoablation enables treatment of breast cancer without a surgical scalpel or tissue removal, effectively killing the tumor in place. This promises many clinical and quality of life benefits for patients. We hope to prove them in this study," states Dr. Gold.
Dr. Fine adds, "Improved screening allows physicians to identify breast cancer earlier, when it's smaller. Advances in molecular profiling (tumor biology) help us better determine which breast cancers have higher or lower risks of recurrence. We can then individualize approaches to treatment. Now with the ICE3 study, we may have the first effective non-surgical treatment option for some low risk patients."
IceCure's clinical trial is recruiting and following women aged 65 years and older diagnosed with luminal A breast tumors measuring less than 1.5 centimeters in diameter. These patients will also receive adjunctive therapy based on local standards of care. Developed by an esteemed and diverse scientific advisory board, the trial is underway at 20 carefully selected sites throughout the United States. These sites already have experience utilizing IceSense3 consoles for the treatment of benign breast tumors, including fibroadenomas. As a market leader in cryoablation treatment of breast disease, IceCure will utilize its resources to provide clinical and technological support to sites during the cancer trial.
"Our study employs the latest generation technology, ensuring that cryoablation is being performed with the coldest, fastest, and most stable equipment available on the market today," said Hezi Himelfarb, CEO. "This study is the next step in IceCure's goal of making non-surgical treatment of breast disease an accepted alternative to the invasive, expensive, surgical approaches today."
The ICE3 trial has also accelerated IceCure's commercialization efforts. With treatment of carefully selected cancer patients in view, the market is responding with increased interest in IceCure's proprietary IceSense3 technology. The initiation of the ICE3 trial, combined with the recent evolution of IceCure's business model, establishes the company as the technological leader in the field of breast cancer treatment using minimally invasive, non-surgical cryoablation.
Cryoablation involves use of extremely low temperatures, applied in a targeted manner to "ablate" or destroy tissue in place. The treatment provides numerous benefits compared to traditional surgical removal of tumors. Cryoablation can be performed in a physician's office with the use of local anesthetic and ultrasound. Additionally, because no tissue is removed, patients endure minimal cosmetic impact with little-to-no scarring or permanent disfigurement. Further, cryoablation is an economically sound alternative to costly surgeries.
About IceCure Medical
IceCure Medical is a medical device company that develops and markets minimally invasive, office-based cryoablation treatment solutions for women's health. Its support of patients and physicians through its Breast Health Options advocacy program embodies its firm commitment to affordable care. The proprietary IceSense3 Cryoablation System offers a comfortable, ultrasound-guided procedure to treat breast tumors. The company was founded in 2006 with main offices in Caesarea, Israel and U.S. headquarters in Memphis, TN. For more information, visit www.icecure-medical.com, www.breasthealthoptions.com, and @CoverCryo on Twitter.
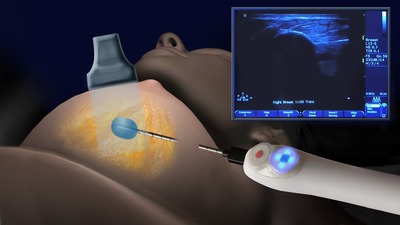
Photo - http://photos.prnewswire.com/prnh/20141021/153279
Photo - http://photos.prnewswire.com/prnh/20140501/83165
To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/national-multi-center-clinical-trial-of-non-surgical-breast-cancer-treatment-enrolls-first-patients-120118156.html
SOURCE IceCure Medical, Inc.
