GeoVax Labs Inc. Reports Progress With Hemorrhagic Fever Vaccine Program
EINPresswire.com -- ATLANTA, GA -- (Marketwired) -- 07/27/16 -- GeoVax Labs, Inc. (OTCQB: GOVX), a biotechnology company developing human vaccines, today provided an update on its Tetravalent Hemorrhagic Fever Vaccine (THV) program, including a demonstration that its vaccines produce non-infectious virus-like particles (VLPs) for each of the four virus targets -- Ebola-Zaire, Ebola-Sudan, Marburg, and Lassa.
GeoVax's vaccine (GOVX- E303) is based on the Company's novel Modified Vaccinia Ankara (MVA) VLP platform, which generates noninfectious VLPs. VLPs, expressed by MVA, form the base for a highly effective vaccine, because they can be engineered to display native forms of membrane-anchored viral envelope glycoproteins that mimic a natural infection. These native proteins trigger the body to produce antibody responses that are highly effective in their ability to block virus infections. Moreover, VLPs generated by GeoVax's vaccines are produced in vivo (in the very person being vaccinated) and do not have to be purified, thereby reducing manufacturing costs.
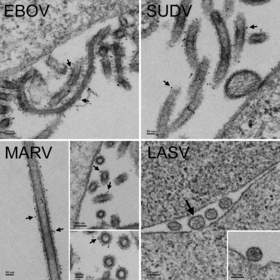
Thin section electron micrographs of MVA-VLP vaccines for Zaire (EBOV), Sudan (SUDV), Marburg (MARV) and Lassa (LASV).
GeoVax has previously demonstrated VLP formation as well as 100% protection in non-human primates after single, or prime/boost, inoculations with its Ebola-Zaire vaccine. The Zaire strain of Ebola virus was responsible for the 2014/15 outbreak in West Africa, which resulted in 28,652 infections and 11,325 deaths. The Company now has vaccine constructs against each of the three additional hemorrhagic fever targets and has demonstrated VLP production for each target infection. GeoVax's vaccines are being developed for use as either individual vaccines in epidemic or biothreat situations or combined as a single tetravalent vaccine for protection of the millions of individuals who live in at-risk areas, travelers, military personnel, and healthcare workers.
Thin section electron micrographs of MVA-VLP vaccines for Zaire (EBOV), Sudan (SUDV), Marburg (MARV) and Lassa (LASV). Non-infectious VLPs budding from the surface of the cells resemble native virions. Immunogold staining was performed using antibodies specific to the glycoproteins of Zaire, Sudan and Marburg viruses (see arrows for examples of gold particles). Lassa VLPs (not immunogold stained) are 100-200 nm in diameter and contain granular structures similar in size to cellular ribosomes, 10 ~ 20 nm (large arrow points to the largest VLP in a cluster of four).
Farshad Guirakhoo, Ph.D., GeoVax's Senior Vice President of Research and Development, commented, "Hemorrhagic fevers caused by filoviruses (Ebola, Sudan, and Marburg viruses) and arenaviruses, such as Lassa virus, occur in an overlapping geographical region in West Africa, putting as many as 200 million people at risk of infection with no licensed vaccines or effective therapeutics available. Lassa virus is endemic and causes severe and often fatal hemorrhagic illness with as many as 67,000 deaths per year. Although many consider MVA a boosting, not a priming, vaccine, our MVA-expressed VLPs are in fact outstanding priming vaccines. In non-human primates, we have demonstrated that a single dose of the GeoVax Ebola-Zaire vaccine elicits complete protective immunity; an important attribute for a vaccine which can be used for travelers or to stem an ongoing epidemic/endemic situation."
Robert McNally, Ph.D., GeoVax's President and CEO, said, "The lessons learned from the 2014/15 Ebola outbreak indicate that the urbanization of Africa contributes to the rapid spread of emergent viruses. If not contained, they can cause global epidemics. The commercial opportunity for our HFV vaccine program is very attractive, particularly for Lassa fever, given the endemic nature of the virus. Currently, ribavirin is used for Lassa fever treatment, but it seems to be effective only if given early on in the course of clinical illness. These viruses could also be intentionally used as biological warfare agents, which makes vaccine development against these agents of high interest to the U.S. Department of Defense and related agencies."
About GeoVax
GeoVax Labs, Inc., is a clinical-stage biotechnology company developing human vaccines against infectious diseases using its MVA-VLP vaccine platform. The Company's development programs are focused on vaccines against HIV, Zika Virus, and hemorrhagic fever viruses (Ebola, Sudan, Marburg, Lassa). GeoVax also recently began programs to evaluate the use of its MVA-VLP platform in cancer immunotherapy, and for therapeutic use in chronic Hepatitis B infections. GeoVax's vaccine platform supports in vivo production of non-infectious VLPs from the cells of the very person receiving the vaccine, mimicking a natural infection, stimulating both the humoral and cellular arms of the immune system to recognize, prevent, and control the target infection. For more information, visit www.geovax.com.
Forward-Looking Statements
Certain statements in this document are "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act. These statements are based on management's current expectations and are subject to uncertainty and changes in circumstances. Actual results may differ materially from those included in these statements due to a variety of factors, including whether: GeoVax can develop and manufacture its vaccines with the desired characteristics in a timely manner, GeoVax's vaccines will be safe for human use, GeoVax's vaccines will effectively prevent targeted infections in humans, GeoVax's vaccines will receive regulatory approvals necessary to be licensed and marketed, GeoVax raises required capital to complete vaccine development, there is development of competitive products that may be more effective or easier to use than GeoVax's products, GeoVax will be able to enter into favorable manufacturing and distribution agreements, and other factors, over which GeoVax has no control. GeoVax assumes no obligation to update these forward-looking statements, and does not intend to do so. More information about these factors is contained in GeoVax's filings with the Securities and Exchange Commission including those set forth at "Risk Factors" in GeoVax's Form 10-K.
Image Available: http://www2.marketwire.com/mw/frame_mw?attachid=3037790
GeoVax Labs, Inc.
Robert T. McNally, Ph.D.
investor@geovax.com
678-384-7220
