Diabetes Research Institute Foundation Release: First Type 1 Diabetes Patient In Europe Is Free From Insulin Therapy After Undergoing Diabetes Research Institute's Biohub Transplant Technique
MIAMI, June 9, 2016 /PRNewswire-USNewswire/ -- A 41-year-old man who was diagnosed with type 1 diabetes at age 11 has become the first patient in Europe to discontinue insulin therapy after receiving a transplant of pancreatic islet cells using an innovative technique developed by the Diabetes Research Institute (DRI) at the University of Miami. Following the DRI's BioHub protocol, Drs. Federico Bertuzzi, head of the Islet Transplant Program, Mario Marazzi, head of the Tissue Therapy Unit, Luciano De Carlis, director of General Surgery and Transplantation, and collaborators at Ospedale Niguarda Ca' Granda in Milan, Italy, transplanted the insulin-producing cells within a biological scaffold engineered onto the surface of the omentum, a highly vascularized tissue covering abdominal organs. This successful post-transplant outcome confirms the initial results achieved by the Diabetes Research Institute in August of 2015.
Ospedale Niguarda Ca' Granda is a member of the worldwide Diabetes Research Institute Federation, a global alliance of researchers and medical centers that collaborate and share promising findings in order to accelerate research toward a cure for diabetes. After completion of the first BioHub transplant, DRI researchers shared their transplant protocol with DRI Federation members with the goal replicating these results quickly to help the millions living with type 1 diabetes around the world.
"I warmly congratulate the team of Niguarda, the first team of the DRI Federation in Europe and in the world to have confirmed the initial results achieved last year in Miami. This tissue engineering technique will be essential for allowing clinical testing of new technologies for preventing the use of anti-rejection drugs which currently limit the applicability of islet transplantation to the most severe cases of type 1 diabetes," said Camillo Ricordi, M.D., director of the DRI and the Stacy Joy Goodman Professor of Surgery, Distinguished Professor of Medicine, Professor of Biomedical Engineering, Microbiology and Immunology at the University of Miami Miller School. Dr. Ricordi also serves as director of the DRI's Cell Transplant Center.
Dr. Ricordi and collaborators from the DRI at UM have been providing assistance to selected teams worldwide by sharing protocols, equipment and through the DRI's telescience platform, which enables researchers in different parts of the world to "virtually" work together side by side.
"Our team is building on decades of progress in clinical islet transplantation toward the development of the DRI BioHub, a bioengineered mini organ that mimics the native pancreas to restore natural insulin production in people with type 1 diabetes," said Dr. Ricordi.
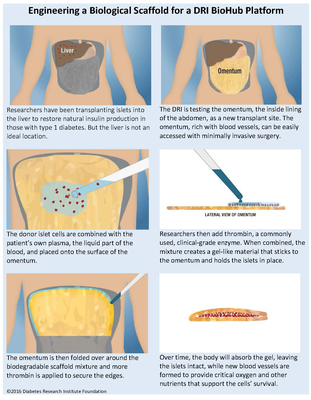
In this latest procedure, as was done in Miami, the donor islets were implanted within a biodegradable scaffold, one of the platforms for a DRI BioHub, made by combining the patient's own blood plasma with thrombin, a commonly used, clinical-grade enzyme. Together, these substances create a gel-like material that sticks to the omentum and holds the islets in place. The omentum is then folded over around the biodegradable (biological) scaffold mixture. Over time, the body will absorb the gel, leaving the islets intact, while new blood vessels are formed to provide critical oxygen and other nutrients that support the cells' survival.
In type 1 diabetes, the insulin-producing islets cells of the pancreas have been mistakenly destroyed by the immune system, requiring patients to manage their blood sugar levels through a daily regimen of insulin therapy. Islet transplantation has allowed some patients to live without the need for insulin injections after receiving a transplant of donor cells. Some patients who have received islet transplants at the DRI have been insulin independent for more than a decade, as DRI researchers have published.
Currently, islet cells are infused into the liver, but many of the cells do not survive in that environment. Most importantly, the reason for developing this alternative site is that the BioHub technology will eventually allow for the introduction of additional technologies and components to eliminate the need for anti-rejection drugs. This FDA-approved Phase I/II BioHub clinical trial is testing the omentum as an alternative transplant site.
About the Diabetes Research Institute
The Diabetes Research Institute at the University of Miami Miller School of Medicine leads the world in cure-focused research. As the largest and most comprehensive research center dedicated to curing diabetes, the DRI is aggressively working to develop a biological cure by restoring natural insulin production and normalizing blood sugar levels without imposing other risks. Researchers have already shown that transplanted islet cells allow patients to live without the need for insulin therapy. The DRI is now building upon these promising outcomes by developing the DRI BioHub and is testing various BioHub platforms in preclinical and clinical studies. For more information, please visit DiabetesResearch.org or call 800-321-3437. You can tweet DRI at @Diabetes_DRI.

Photo - http://photos.prnewswire.com/prnh/20160609/377653-INFO
Logo - http://photos.prnewswire.com/prnh/20120405/DC83112LOGO
To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/first-type-1-diabetes-patient-in-europe-is-free-from-insulin-therapy-after-undergoing-diabetes-research-institutes-biohub-transplant-technique-300282581.html
SOURCE Diabetes Research Institute Foundation

