Benitec Releases Pivotal Data In An In Vivo Hepatitis B Model
SYDNEY, Dec. 20, 2016 /PRNewswire/ -- Benitec Biopharma Limited (ASX:BLT; NASDAQ: BNTC; NASDAQ: BNTCW) today announced significant progress on use of the company's technology for development of a ddRNAi based therapeutic for the treatment of hepatitis B virus (HBV). A combination approach of using a single administration of the DNA-directed RNA interference (ddRNAi) agents BB-101, BB-102 or BB-103 with current standard of care agents used to treat the disease, demonstrates a robust and sustained suppression of HBV in an in vivo model.
Benitec's ddRNAi technology is a unique combination of gene silencing using RNA interference coupled with the long term therapeutic activity of gene therapy vectors. For the HBV program, the lead candidates are comprised of an adeno associated virus capsid (AAV8) and a recombinant DNA cassette engineered to express steady state levels of three short hairpin RNA (shRNA) that inhibit HBV viral RNA at three regions well conserved across all major genotypes.
This current in vivo study assessed the activity of BB-101, BB-102 and BB-103 in the PhoenixBio (PXB) mouse model, in which a substantial portion of the mouse liver cells have been replaced with human hepatocytes making the animals susceptible to HBV infection. BB-101 is comprised of a single stranded recombinant DNA vector expressing three anti-HBV shRNA. BB-102 is similar to BB-101, with the exception that the recombinant genome is packaged as a self-complementary, double stranded DNA. BB-103 is a next generation vector in which the anti-HBV shRNA have been modeled into miRNA backbones for expression from wildtype pol III promoters.
The ddRNAi components were administered only once, at the beginning of treatment, and anti-HBV activity was monitored over the course of 13 weeks by following serum HBV DNA, HBsAg and HBeAg on a weekly basis. At the conclusion of the study, intracellular HBV DNA as well as cccDNA was quantified. In combination studies, a single dose of ddRNAi vectors was administered with daily entecavir (ETV), a nucleoside reverse transcriptase inhibitor, or a pegylated interferon agent that was administered twice a week for the duration of the study.
Benitec's Chief Scientific Officer, Dr. David Suhy said, "It is remarkable that these ddRNAi treatments, administered as a single infusion on top of an existing treatment regimen have this magnitude of impact on the viral burden in this model of HBV infection. With a high degree of confidence in our efficacy studies, we look forward to take the next steps of being able to move these compounds towards human clinical testing. To this end, we anticipate meeting with a number of regulatory agencies in early 2017."
The key findings are:
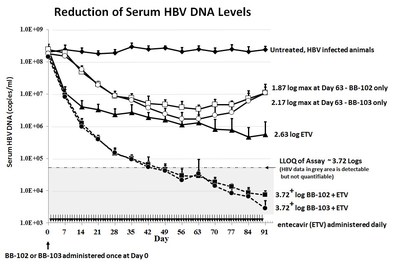
- Dosed individually in the absence of other anti-viral drugs, BB-103 and BB-102 resulted in corresponding maximum drop of serum HBV DNA levels at 2.17 log and 1.87 log reduction. A modest rebound of HBV DNA levels were noted following 56 days of treatment. A treatment arm consisting only of daily entecavir resulted in in a 2.63 log drop in serum HBV DNA levels.
- In combination with daily entecavir, a single dose of BB-103 and BB-102 dropped the serum HBV DNA levels below 3.72 log, the lower limit of quantification (LLOQ) for the assay. The LLOQ represents the lowest value that can result in accurate quantification of HBV DNA levels. Although HBV DNA is detectable below this level, it cannot be quantified. The reduction in viral burden continued to diminish until the end of the the 91 day experiment.
- BB-103 + entecavir and BB-102 + entecavir dropped HBsAg levels, a known contributor to immunosuppression and HBV chronicity, by 2.14 log and 1.86 log. Treatment with entecavir only dropped HBsAg levels by 0.46 log.
- BB-103 + ETV and BB-102 + ETV dropped HBeAg levels by 1.90 log and 1.42 log respectively. Treatment with entecavir only dropped HBsAg levels by 0.37 log.
Treatment | Log Reduction of Serum HBV DNA | Log Reduction of HBsAg | Log Reduction of HBeAg | |
Control groups | entecavir (ETV) 6 mg/kg daily | 2.63 | 0.46 | 0.37 |
pegylated interferon 30 mg/kg twice weekly | 2.41 | 0.96 | 1.09 | |
Single administration of ddRNAi | BB-102 2e13 vg/kg | 1.87 max at Day 63 | 1.75 max at Day 70 | 1.17 max at Day 56 |
BB-103 2e13 vg/kg | 2.17 max at Day 63 | 1.94 max at Day 70 | 1.61 max at Day 56 | |
Single administration of ddRNAi with daily entecavir | BB-102 + ETV | * 3.72 + | 1.86 | 1.42 |
BB-103 + ETV | * 3.72 + | 2.14 | 1.90 |
* The LLOQ for the serum HBV DNA assay is 4e4 copies/ml or approximately a 3.72 log drop.
A full presentation of the data sets will occur at key upcoming liver conferences in 2017.
For further information regarding Benitec and its activities, please contact the persons below, or visit the Benitec website at www.benitec.com
Australia Investor Relations | United States Investor Relations |
Market Eye Orla Keegan Director Tel: +61 (2) 8097 1201 Email: orla.keegan@marketeye.com.au | M Group Strategic Communications Jay Morakis Managing Director Tel: +1 212.266.0190 Email: jmorakis@MGroupSC.com |
About Benitec Biopharma Limited:
Benitec Biopharma Limited (ASX: BLT; NASDAQ: BNTC; NASDAQ: BNTCW) is a biotechnology company developing innovative therapeutics based on its patented gene-silencing technology called ddRNAi or 'expressed RNAi'. Based in Sydney, Australia with laboratories in Hayward, California (USA), and collaborators and licensees around the world, the company is developing ddRNAi-based therapeutics for chronic and life-threatening human conditions including hepatitis B, wet age-related macular degeneration and OPMD. Benitec has also licensed ddRNAi to other biopharmaceutical companies for applications including HIV/AIDS, Huntington's Disease, chronic neuropathic pain, cancer immunotherapy and retinitis pigmentosa.
Safe Harbor Statement:
This press release contains "forward-looking statements" within the meaning of section 27A of the US Securities Act of 1933 and section 21E of the US Securities Exchange Act of 1934. Any forward-looking statements that may be in the press release are subject to risks and uncertainties relating to the difficulties in Benitec's plans to develop and commercialise its product candidates, the timing of the initiation and completion of preclinical and clinical trials, the timing of patient enrolment and dosing in clinical trials, the timing of expected regulatory filings, the clinical utility and potential attributes and benefits of ddRNAi and Benitec's product candidates, potential future out-licenses and collaborations, the intellectual property position and the ability to procure additional sources of financing. Accordingly, you should not rely on those forward-looking statements as a prediction of actual future results.
To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/benitec-releases-pivotal-data-in-an-in-vivo-hepatitis-b-model-300381842.html
SOURCE Benitec Biopharma Limited

