Bayer HealthCare Submits Biologics License Application For BAY 81-8973 For The Treatment Of Hemophilia A In Adults And Children
WHIPPANY, N.J., Dec. 17, 2014 /PRNewswire/ -- Bayer HealthCare has submitted a Biologics License Application (BLA) to the U.S. Food and Drug Administration (FDA) seeking approval for BAY 81-8973, a recombinant Factor VIII (rFVIII) compound, for the treatment of hemophilia A in children and adults. The submission was based on results from the LEOPOLD (Long-Term Efficacy Open-Label Program in Severe Hemophilia A Disease) clinical trials, which evaluated BAY 81-8973 in adults and children using two- and three-times-per-week prophylaxis dosing regimens.

"The LEOPOLD study program and the submission of BAY 81-8973 are a demonstration of Bayer's continued, longstanding commitment to the hemophilia A community," said Dario Mirski, M.D., Vice President and Head, U.S. Medical Affairs, Bayer HealthCare Pharmaceuticals.
BAY 81-8973 is an investigational agent and is not approved by the United States Food and Drug Administration (FDA), the European Medicines Agency (EMA) or other health authorities. An application for marketing authorization for BAY 81-8973 was submitted to the EMA in December 2014 for approval of the same indication in the European Union.
About LEOPOLD
The LEOPOLD (Long-Term Efficacy Open-Label Program in Severe Hemophilia A Disease) Clinical Development Program consists of three multinational clinical trials designed to evaluate the pharmacokinetics, efficacy, and safety of BAY 81-8973 in subjects with severe hemophilia A (<1% FVIII:C).
LEOPOLD I is an open-label, randomized study. Part A studied the pharmacokinetics of BAY 81-8973. Part B evaluated the safety, tolerability and efficacy of prophylaxis with BAY 81-8973. Part C evaluated hemostatic outcome during major surgery. Included were male patients (ages 12-65 years) with severe hemophilia A (<1% FVIII:C). The primary endpoint of Part B was annualized number of all bleeds over 12 months.
LEOPOLD II is a randomized, cross-over, open-label trial also in male subjects aged 12 to 65 years. In this Phase II/III study, 80 subjects were randomized to receive BAY 81-8973 either as a low-dose prophylaxis regimen (20-30 IU/kg; n=28) twice-per-week, high-dose prophylaxis (30-40 IU/kg; n=31) three-times-per-week, or on-demand (n=21). The primary objective was to demonstrate the superiority of prophylaxis versus on-demand therapy, with the primary endpoint being bleeding frequency at 12 months.
LEOPOLD Kids is a two-part, open-label, non-randomized phase III study. Part A is designed to evaluate the efficacy and safety of BAY81-8973 for prophylaxis, treatment of bleeds, and surgical management in previously treated children <=12 years of age. Part B of the study, which involves previously untreated patients (PUPs), is ongoing.
About Hemophilia A
Hemophilia A, also known as factor VIII deficiency or classic hemophilia, is a largely inherited bleeding disorder in which one of the proteins needed to form blood clots in the body is missing or reduced. Hemophilia A is the most common type of hemophilia and is characterized by prolonged or spontaneous bleeding, especially into the joints, muscles or internal organs.
About Hematology at Bayer HealthCare
Bayer HealthCare is committed to delivering science for a better life by advancing a portfolio of treatments. Hematology at Bayer HealthCare includes numerous compounds in various stages of development for hemophilia, sickle cell anemia, and other blood and bleeding disorders. Together, these compounds reflect the company's commitment to research and development, prioritizing specific targets for intervention with the potential to improve the way that rare blood and bleeding disorders are treated.
About Bayer HealthCare Pharmaceuticals Inc.
Bayer HealthCare Pharmaceuticals Inc. is the U.S.-based pharmaceuticals business of Bayer HealthCare LLC, a subsidiary of Bayer AG. Bayer HealthCare is one of the world's leading, innovative companies in the healthcare and medical products industry, and combines the activities of the Animal Health, Consumer Care, Medical Care, and Pharmaceuticals divisions. As a specialty pharmaceutical company, Bayer HealthCare provides products for General Medicine, Hematology, Neurology, Oncology and Women's Healthcare. The company's aim is to discover and manufacture products that will improve human health worldwide by diagnosing, preventing and treating diseases.
Bayer® and the Bayer Cross® are registered trademarks of Bayer.
Forward-Looking Statements
This news release may contain forward-looking statements based on current assumptions and forecasts made by Bayer Group or subgroup management. Various known and unknown risks, uncertainties and other factors could lead to material differences between the actual future results, financial situation, development or performance of the company and the estimates given here. These factors include those discussed in Bayer's public reports which are available on the Bayer website at www.bayer.com. The company assumes no liability whatsoever to update these forward-looking statements or to conform them to future events or developments.


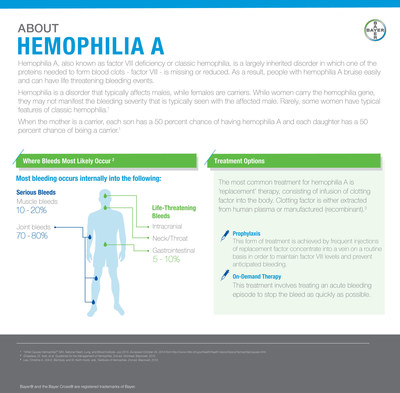

Photo - http://photos.prnewswire.com/prnh/20141217/164909
Photo - http://photos.prnewswire.com/prnh/20141217/164906
Photo - http://photos.prnewswire.com/prnh/20141217/164908
Photo - http://photos.prnewswire.com/prnh/20141217/164910-INFO
Logo - http://photos.prnewswire.com/prnh/20140312/NY79226LOGO
To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/bayer-submits-biologics-license-application-for-bay-81-8973-for-the-treatment-of-hemophilia-a-in-adults-and-children-300011070.html
SOURCE Bayer HealthCare
