Avita Medical Initiates European Trial Using ReCell in the Management of Chronic Lower Limb Ulcers
NORTHRIDGE, CA and CAMBRIDGE, UNITED KINGDOM--(Marketwire - November 20, 2012) -
| Highlighted Links |
Avita Medical website |
- UP TO FIVE EUROPEAN CENTERS TO PARTICIPATE
- PRELIMINARY STUDIES IN TREATMENT OF VENOUS LEG ULCERS YIELD POSITIVE RESULTS: DEMONSTRATE ACCELERATED HEALING AND REDUCED PAIN AS COMPARED WITH CURRENT STANDARD OF CARE
- SUBSTANTIAL MARKET; US ANNUAL LOWER LIMB ULCER TREATMENT COSTS $25 BILLION
Regenerative medicine company Avita Medical Ltd. (ASX: AVH) (OTCQX: AVMXY) has initiated a multicenter randomized control study on the use of ReCell® Spray-On Skin® in the treatment of venous leg ulcers. Up to five European centers from countries including the United Kingdom, Germany, France and Denmark will participate in the study.
Lower limb ulcers (LLUs), which include venous leg ulcers and diabetic foot ulcers, are a major healthcare problem in developed countries due to their prevalence, high cost of treatment and significant impact on patient quality of life. LLUs afflict nearly 1.5% of the general population in OECD countries and up to 3% over the age of 70 years. The expense of treating LLUs imposes a major financial burden on healthcare systems. In the US alone some 6.5 million people suffer from LLUs with associated treatment costs estimated in excess of US$25 billion per annum with similar prevalence and expense documented in the UK, Germany and France. The costs to patients include associated morbidity, pain, lack of mobility and lost work days and wages.
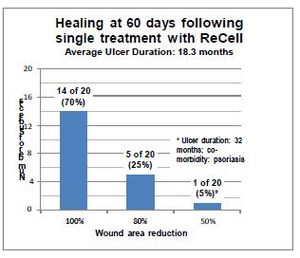
In preliminary open-label studies ReCell was used at four European centers to treat approximately 80 patients suffering from venous leg ulcers and diabetic foot ulcers. Across study centers patients' average age was approximately 70 years, wounds were open an average of approximately 13 months and averaged approximately 21 cm2 in size.
Results of these preliminary studies have been encouraging. Over 70% of patients showed complete healing of the wound within 7-12 weeks following a single treatment with ReCell. With the current standard of care treatment it would be expected that approximately 43% of these wounds would have achieved closure during this period, thus ReCell appears to yield a highly significant positive effect on wound healing. Pain, a critical indicator of quality of life, was reported as being significantly reduced or eliminated within 72 hours following the ReCell treatment.
These data have been presented at congresses and have been submitted as separate papers for publication in peer-reviewed journals.
Given the positive results achieved in preliminary studies, Avita has initiated a randomized control study with leading clinicians at up to 5 European centers. Up to 80 patients will be enrolled in the study. Study protocols have been submitted for approval by the relevant ethics review boards at each center. It is anticipated that enrolment in the study will commence during the 1st quarter of 2013.
Dr. William Dolphin, CEO of Avita Medical, said, "The early results achieved in the treatment of chronic ulcers with ReCell are highly promising. These are hard-to-heal ulcers that pose an enormous burden on healthcare systems and seriously impact on patient's quality of life.
"ReCell is approved for use in Europe, Australia and other markets with over 4500 procedures conducted to date, mainly for burns. Improved leg ulcer treatment will provide tremendous relief for ulcer patients, potentially deliver significant cost savings to financially stressed healthcare systems and may open additional very large markets for ReCell."
ABOUT AVITA MEDICAL LTD.
Avita Medical (http://www.avitamedical.com/) develops and distributes regenerative and tissue-engineered products for the treatment of a broad range of wounds, scars and skin defects. Avita's patented and proprietary tissue-culture, collection and application technology provides innovative treatment solutions derived from a patient's own skin. The company's lead product, ReCell® Spray-On Skin™, is used in a wide variety of burns, plastic, reconstructive and cosmetic procedures. ReCell is patented, CE-marked for Europe, TGA-registered in Australia, and SFDA-cleared in China. ReCell is not available for sale in the United States; in the US ReCell is an investigational device limited by federal law to investigational use. An FDA trial on the safety and efficacy of ReCell is in process.
Contact:
Avita Medical Ltd.
Stella Sung, Ph.D.
Business Development Officer
Phone:+1 818-356-9400
Email: ssung@avitamedical.com
Investor Relations:
ProActive Capital Resources Group, LLC
Jeff Ramson
CEO
641 Lexington Avenue, 6th Floor
New York, NY. 10022
Phone:+1 646-863-6893
Email: jramson@proactivecapitalgroup.com

 Digg this
Digg this Bookmark with del.icio.us
Bookmark with del.icio.us Add to Newsvine
Add to Newsvine