Applied Spectral Imaging (ASI)'s Advanced Algorithms Automatically Interpret PD-L1 Assays
CARLSBAD, California, May 24, 2017 /PRNewswire/ -- Applied Spectral Imaging (ASI), a global leader in computer-assisted image analysis platforms, introduces HiPath for analysis of PD-L1 assays.
HiPath, ASI's advanced IHC platform, is a pioneer in digital pathology solutions that support PD-L1 analysis, for research use. It determines PD-L1 protein expression by calculating the percentage of viable tumor cells showing partial or complete membrane staining, and automatically provides the tumor proportion score (TPS).
PD-L1 is seen as the current breakthrough therapeutic target in immuno-oncology and has become a key factor in determining the treatment for patients with non-small cell lung cancer (NSCLC), the most common type of cancer which accounts for 13% of the total number of cancer cases in the US. The significant success of PD-1/PD-L1 blockades in lung cancer has led to the FDA approval of revolutionary immunotherapy drugs, and PD-L1 assays are becoming standard care for all patients with a suspected lung cancer.
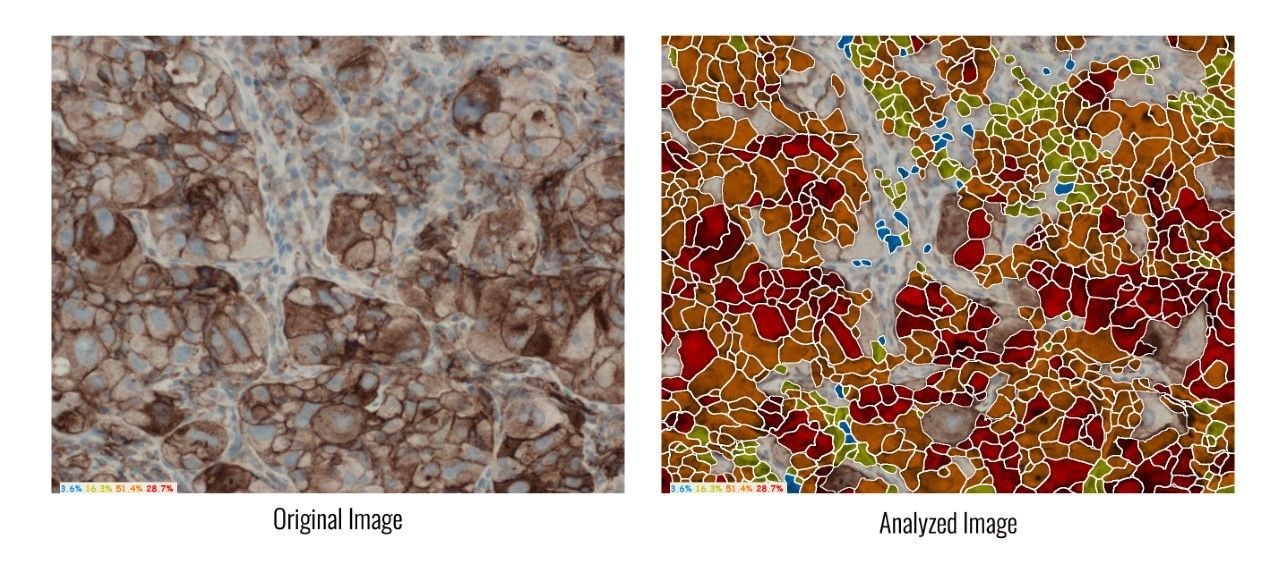
Photo - https://mma.prnewswire.com/media/510169/ASI.jpg?w=1600
"We continuously strive to improve patient care, through cutting-edge technology that assists pathologists in providing accurate and standardized results for even the toughest cases," said Limor Shiposh, ASI's CEO.
HiPath provides digital automation of IHC tissue analysis. It allows pathologists to capture images, receive computer-aided scoring, and generate automatic reports with complete case statistics. HiPath provides accurate, reproducible and consistent results, while integrating seamlessly with pathologists' existing microscope and workflow. It is FDA cleared for ER, PR, HER2/neu, and Ki-67, yet supports all nuclear and membrane stains.
About ASI
ASI is a global leader in the development of imaging solutions, supporting fluorescent, brightfield and spectral image-acquisition, for pathology tests, FISH and karyotyping.
The HiPath and GenASIs automated imaging platforms for pathology and genetic analysis provide advanced diagnostic aids for pathologists and cytogeneticists, with reproducible and standardized results. ASI platforms support manual and automatic scanning for a wide range of workflows and applications, to best suit the needs, size and budget of any lab.
ASI's applications are FDA cleared for BandView, FISHView, CEP XY, UroVysion, ALK, HER2/neu FISH and IHC Family for: HER2, ER, PR and Ki67.
ASI serves clinical laboratories, life science companies and research institutions in over 50 countries.
For More Information Please Contact
Applied Spectral Imaging
Phone: +1 (760) 929-2840
E-mail: info@spectral-imaging.com
www.appliedspectralimaging.com
To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/asis-advanced-algorithms-automatically-interpret-pd-l1-assays-300463131.html
SOURCE Applied Spectral Imaging (ASI)

