Adapt Pharma To Launch Program To Raise Awareness, Expand Access And Facilitate Product Experience Of NARCAN (Naloxone Hcl) Nasal Spray - A New Treatment For Opioid Overdose
DUBLIN, July 12, 2016 /PRNewswire/ -- Adapt Pharma, Limited, (www.adaptpharma.com) today announced that it has launched a program providing a donation of 25,000 cartons (50,000 doses) of NARCAN® (naloxone HCl) Nasal Spray 4mg, the first and only FDA-approved naloxone nasal spray, to community partners. Specifically, Adapt has partnered with Police Assisted Addiction and Recovery Initiative (P.A.A.R.I.), the Harm Reduction Coalition (HRC), and the Clinton Health Matters Initiative, to allocate donated product to their constituents that may benefit from the program.
In addition, Adapt will offer donated product to the city law enforcement agencies across the country who have been hit hardest by opioid-related overdoses to assist them in their fight against opioid overdose. The program allocations are expected to be completed by the end of July 2016.
"Having launched our recently FDA-approved NARCAN® Nasal Spray, this donation is intended to both raise awareness and expand access and user experience with this ready-to-use emergency opioid overdose treatment," said Seamus Mulligan, Chairman and CEO of Adapt Pharma. "Potential users include first responders, such as police officers, as well as patients' friends, family and loved ones. Each of these partner organizations brings unique resources and outlets to connect the donated product with these potential overdose witnesses."
"Harm reduction programs are at the forefront of equipping people at risk of overdose and their loved ones with knowledge and tools to save lives," said Daniel Raymond, Policy Director for the Harm Reduction Coalition. "Communities look to harm reduction programs for training and leadership on naloxone strategies. This donation will help our efforts."
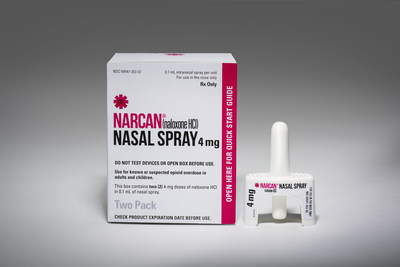
NARCAN® Nasal Spray is the first and only FDA-approved naloxone nasal spray for the emergency treatment of opioid-, fentanyl- and heroin-related overdose. It is now available as a ready-to-use, needle-free, 4 mg concentrated dose of naloxone in a single spray. As the first and only FDA-approved naloxone nasal spray, NARCAN® Nasal Spray provides a ready-to-use alternative to currently available opioid overdose emergency treatments. NARCAN® Nasal Spray is not a substitute for emergency medical care. Please see Indications and Important Safety Information below.
"Adapt Pharma's donation of NARCAN Nasal Spray is an important next step in the Clinton Health Matters Initiative's work to provide a predictable and affordable supply of potentially life-saving naloxone to our community partners across the country," said Rain Henderson, CEO of the Clinton Health Matters Initiative. "Although there is much work to be done in addressing the opioid epidemic, Adapt Pharma's donation can help expand the use of opioid overdose treatment for at risk populations."
"While a key component of opioid abuse is treatment and rehabilitation, many users need that second chance at life to enter into long-term addiction treatment and recovery," said John E. Rosenthal, Co-founder, Chairman of the Board of Directors of Police Assisted Addiction and Recovery Initiative (P.A.A.R.I.) "From police, to harm reduction groups, to NGOs and so on, increased access to naloxone across all stakeholders can give people that second chance and help reduce the number of opioid-related deaths in the United States."
"As the face of opioid abuse has evolved, and new, more powerful opioids, such as fentanyl, are hitting the streets, the U.S. government and supporting agencies have strengthened the call for a broad array of tools," said Mike Kelly, President US Operations of Adapt Pharma. "Strides toward broadening and improving access to opioid overdose reversal medications are one key element in making an impact today."
Beginning July 12, product will be distributed directly to the qualified recipients. These recipients will be determined by P.A.A.R.I., HRC and the Clinton Health Matters Initiative. Each of these organizations have a specific set of criteria to determine the appropriate recipients. Adapt will ensure each recipient qualifies under the terms and conditions set forth to govern this program. This experience program will be realized by the end of July 2016.
Indications and Important Safety Information about NARCAN® Nasal Spray
ABOUT NARCAN® (naloxone HCl) NASAL SPRAY
NARCAN® Nasal Spray is indicated for the emergency treatment of known or suspected opioid overdose, as manifested by respiratory and/or central nervous system depression.
NARCAN® Nasal Spray is intended for immediate administration as emergency therapy in settings where opioids may be present.
NARCAN® Nasal Spray is not a substitute for emergency medical care. Always seek emergency medical assistance in the event of a suspected, potentially life-threatening opioid emergency after administration of the first dose of NARCAN® nasal spray.
If the desired response is not obtained after 2 or 3 minutes, administer an additional dose of NARCAN® Nasal Spray using a new NARCAN® Nasal Spray. If the patient responds to NARCAN® Nasal Spray and relapses back into respiratory depression before emergency assistance arrives, administer an additional dose and continue surveillance of the patient. If there is still no response and additional doses are available, administer additional doses of NARCAN® Nasal Spray every 2 to 3 minutes using a new NARCAN® Nasal Spray with each dose until emergency medical assistance arrives. Additional supportive and/or resuscitative measures may be helpful while awaiting emergency medical assistance.
Please see Indications and Important Safety Information below.
Please see full prescribing information for NARCAN® Nasal Spray, available at http://www.NARCANnasalspray.com/pdf/NARCAN-Prescribing-Information.pdf
AVAILABILITY OF NARCAN® NASAL SPRAY
NARCAN® Nasal Spray was made available in February of 2016.
Qualifying group purchasers may source NARCAN® Nasal Spray directly from wholesalers and distributors. To place a pre-order immediately or for assistance in sourcing NARCAN® Nasal Spray please contact Adapt Pharma's dedicated Customer Service Team at 844-4-NARCAN® (844-462-7226) or email customerservice@adaptpharma.com.
NARCAN® NASAL SPRAY INDICATIONS AND IMPORTANT SAFETY INFORMATION
Indications
NARCAN® (naloxone hydrochloride) Nasal Spray is an opioid antagonist indicated for the emergency treatment of known or suspected opioid overdose, as manifested by respiratory and/or central nervous system depression. NARCAN® Nasal Spray is intended for immediate administration as emergency therapy in settings where opioids may be present.
NARCAN® Nasal Spray is not a substitute for emergency medical care.
Important Safety Information
NARCAN® Nasal Spray is contraindicated in patients known to be hypersensitive to naloxone hydrochloride.
Seek emergency medical assistance immediately after initial use, keeping the patient under continued surveillance.
Risk of Recurrent Respiratory and CNS Depression: Due to the duration of action of naloxone relative to the opioid, keep the patient under continued surveillance and administer repeat doses of naloxone using a new nasal spray with each dose, as necessary, while awaiting emergency medical assistance.
Risk of Limited Efficacy with Partial Agonists or Mixed Agonists/Antagonists: Reversal of respiratory depression caused by partial agonists or mixed agonists/antagonists, such as buprenorphine and pentazocine, may be incomplete. Larger or repeat doses may be required.
Precipitation of Severe Opioid Withdrawal: Use in patients who are opioid dependent may precipitate opioid withdrawal characterized by body aches, diarrhea, increased heart rate (tachycardia), fever, runny nose, sneezing, goose bumps (piloerection), sweating, yawning, nausea or vomiting, nervousness, restlessness or irritability, shivering or trembling, abdominal cramps, weakness, and increased blood pressure. In neonates, opioid withdrawal may be life-threatening if not recognized and properly treated and may be characterized by convulsions, excessive crying, and hyperactive reflexes. Monitor for the development of opioid withdrawal.
Risk of Cardiovascular (CV) Effects: Abrupt postoperative reversal of opioid depression may result in adverse CV effects. These events have primarily occurred in patients who had pre-existing CV disorders or received other drugs that may have similar adverse CV effects. Monitor these patients closely in an appropriate healthcare setting after use of naloxone hydrochloride.
The following adverse reactions were observed in a NARCAN Nasal Spray clinical study: increased blood pressure, musculoskeletal pain, headache, nasal dryness, nasal edema, nasal congestion, and nasal inflammation.
See Instructions for Use and full prescribing information in the use of this product, available here: http://www.NARCANnasalspray.com/pdf/NARCAN-Prescribing-Information.pdf.
Additional information, including full prescribing information for NARCAN® Nasal Spray, and important safety information and instructions for use, is also available at www.NARCAN®NasalSpray.com.
To report SUSPECTED ADVERSE REACTIONS, contact Adapt Pharma, Inc. at 1-844-4NARCAN® (1-844-462-7226) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
ABOUT THE CLINTON HEALTH MATTERS INITIATIVE
The Clinton Health Matters Initiative (CHMI) is an initiative of the Clinton Foundation that works to improve the health and well-being of people across the U.S. by activating individuals, communities, and organizations to make meaningful contributions to the health of others. Through strategic partnerships, CHMI constructs national programs around key issue areas in chronic diseases that disproportionately affect underserved populations. By implementing evidence based systems, environmental, and investment strategies, CHMI aims to ultimately reduce the prevalence of preventable diseases and close health inequity and disparity gaps, thus improving the quality of life for people across the U.S. Learn more at www.clintonfoundation.org, or by following the Clinton Foundation on Facebook (facebook.com/clintonfoundation) or on Twitter (twitter.com/clintonfdn).
ABOUT HARM REDUCTION COALITION
Harm Reduction Coalition was founded in 1993 and incorporated in 1994 by a working group of needle exchange providers, advocates and drug users. Today, we are strengthened by an extensive and diverse network of allies who challenge the persistent stigma faced by people who use drugs and advocate for policy and public health reform. Learn more at www.harmreduction.org
ABOUT POLICE ASSISTED ADDICTION AND RECOVERY INITIATIVE (P.A.A.R.I.)
The Police Assisted Addiction and Recovery Initiative (P.A.A.R.I.) was started to support local police departments as they work with opioid addicts. Rather than arrest our way out of the problem of drug addiction, P.A.A.R.I. committed police departments:
- Encourage opioid drug users to seek recovery
- Help distribute lifesaving opioid blocking drugs to prevent and treat overdoses
- Connect addicts with treatment programs and facilities
- Provide resources to other police departments and communities that want to do more to fight the opioid addiction epidemic
Learn more at paariusa.org
ABOUT ADAPT PHARMA
Adapt Pharma is a privately-held pharmaceutical company committed to positively impacting the lives of patients. Adapt Pharma's strategy is to identify, evaluate, selectively acquire and enhance the value of late stage development, and FDA approved, pharmaceutical products. Adapt Pharma's company headquarters is in Dublin, Ireland and its U.S. headquarters is in Radnor, Pennsylvania. For more information, please visit www.adaptpharma.com.
MEDIA CONTACT INFORMATION
Thom Duddy
Executive Director, Communications & Marketing
Adapt Pharma
thomas.duddy@adaptpharma.com
484-532-5470
Clinton Foundation Press Office
Clinton Health Matters Initiative
press@clintonfoundation.org
212-348-0360
John Guilfoil
Police Assisted Addiction and Recovery Initiative (P.A.A.R.I.)
617-993-0003
Daniel Raymond
Policy Director
Harm Reduction Coalition
212-213-6376 ext. 29
©2016 ADAPT Pharma, Inc. NARCAN® is a registered trademark licensed to ADAPT Pharma Operations Limited. ADPT-27-16 ADAPT Pharma, Inc. Radnor, PA.

Photo - http://photos.prnewswire.com/prnh/20160712/388735
Logo - http://photos.prnewswire.com/prnh/20160410/353441LOGO
To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/adapt-pharma-to-launch-program-to-raise-awareness-expand-access-and-facilitate-product-experience-of-narcan-naloxone-hcl-nasal-spray---a-new-treatment-for-opioid-overdose-300297275.html
SOURCE Adapt Pharma

