Lipogems Announces New FDA Clearance
NORCROSS, Ga., Nov. 22, 2016 /PRNewswire/ -- Lipogems, a leading international tissue repair company, today announced the U.S. Food and Drug Administration 510(k) clearance for expanded indications and labeling of The Lipogems® System for adipose tissue transfer.
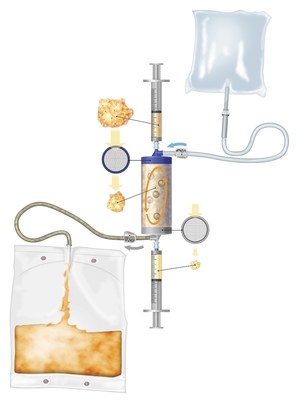
Lipogems' historical offering provides a single-use, disposable device which processes autologous adipose tissue for use as an alternative, and/or as an adjunct, to surgery for filling soft tissue defects and promoting healing in orthopaedics, plastic surgery, and a number of other surgical specialties.
Lipogems' new FDA clearance better defines the indications as harvesting, concentrating and transferring of autologous adipose tissue, and expands the initial indications into the field of arthroscopy (i.e. minimally invasive surgery in joints). In addition, the updated FDA clearance defines that the Lipogems System preserves the cell and tissue micro-architecture of the adipose tissue, eliminates residues of oil emulsion and blood, and provides a tissue that is minimally manipulated in accordance with FDA guidelines for Human Cell and Tissue Products.
"The ability to provide patients with alternative solutions to major surgery throughout their lives is important in helping them to maintain and enjoy a healthy, active lifestyle. This new clearance will give both physicians and patients better clarity on the indications for use of the Lipogems System, which we are confident will have a positive effect on the healing process for patients who are candidates for arthroscopic surgery and those seeking alternatives to major surgery," said Carl Llewellyn, President, Lipogems USA. "We are thankful to the FDA for working with Lipogems to better define our indications, and excited to offer this therapeutic alternative." |
About Lipogems
Lipogems is an international company with US operations headquartered outside Atlanta, Georgia. The company's goal is to partner with healthcare professionals around the world to improve patients' quality of life. The company offers a product and services that impacts orthopedic surgery, arthroscopic surgery, neurosurgery, gastrointestinal and affiliated organ surgery, urological surgery, general surgery, gynecological surgery, thoracic surgery, laparoscopic surgery, and plastic and reconstructive surgery. Lipogems is active in over 25 countries around the world. Email to learn more at info@lipogems.us.com.

Photo - http://photos.prnewswire.com/prnh/20161122/442099
Logo - http://photos.prnewswire.com/prnh/20161122/442098LOGO
To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/lipogems-announces-new-fda-clearance-300367422.html
SOURCE Lipogems

